FIG 2.
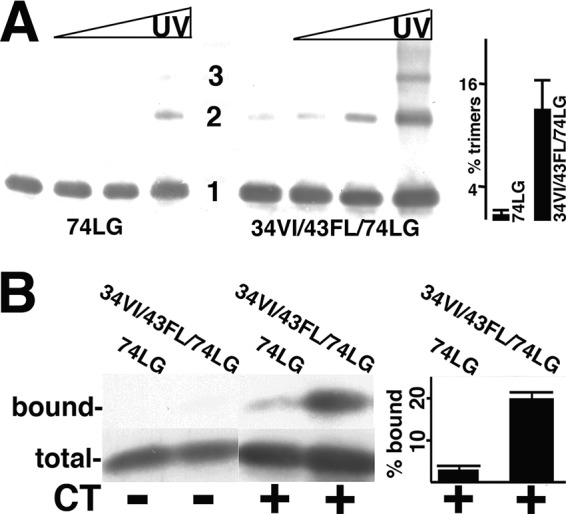
Analysis of MA mutant protein trimerization and CT binding. (A) Purified 74LG or 34VI/43FL/74LG MA proteins at 50 μM concentrations were cross-linked for 0, 1, 3, and 10 min (left to right), separated by electrophoresis, and immunoblotted with an anti-MA antibody. Monomer (1), dimer (2), and trimer (3) sizes were determined by the mobilities of size standards run in parallel. The far-right panel shows the percentage of trimers (relative to monomers) from 10-min cross-linking time points averaged from two separate experiments, with standard deviations as indicated. The P value for the observed difference is 0.0315. (B) The indicated purified MA proteins at 1.5 μM concentrations were incubated with beads coated with glutathione S-transferase (GST) (CT−) or GST-CT (CT+), after which total unbound proteins were collected (total) and beads were washed and eluted to collect bound proteins. Total and bound samples were electrophoretically separated and immunoblotted in parallel for protein detection. Immunoblot bands were quantified densitometrically, and relative binding levels are expressed as percentages of bound versus total MA protein. Calculated percentages are derived from three independent experiments, with standard deviations as indicated. The P value for the observed difference between 74LG and 34VI/43LF/74LG binding to GST-CT is 0.0007.
