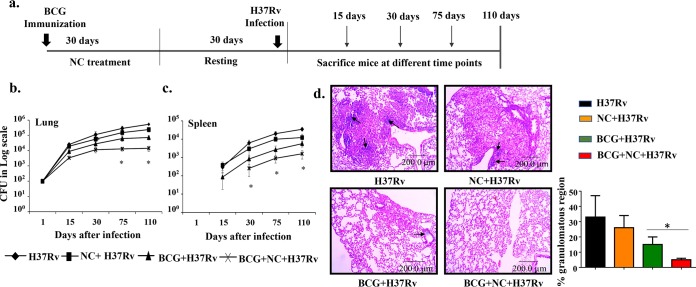
FIG 2.
Nanocurcumin enhances BCG vaccine efficacy in H37Rv-challenged mice. Mice were distributed into 4 groups: (i) naive C57BL/6 mice, (ii) nanocurcumin (NC)-treated mice, (iii) BCG-immunized and PBS-treated mice, and (iv) BCG-immunized and nanocurcumin-treated mice. (a) Schematic representation of the experiment. Mice were immunized with BCG (subcutaneously) and then injected with nanocurcumin for 30 days, followed by a resting period of another 30 days. Mice were then challenged with H37Rv via the aerosol route, with a low-dose inoculum of approximately 110 CFU per mouse. After that, mice were euthanized at various time points (15, 30, 75, and 110 days), and lungs and spleen were harvested and assessed for bacterial burden. (b) CFU counts in lungs at different time intervals. (c) CFU counts in spleen at different time intervals. (d, left) Photomicrographs of lung histological sections (6 μm) of different experimental groups after 75 days of infection, stained with hematoxylin and eosin. Arrows denote the granulomatous region. (Right) Graphical representation of the percentages of granulomatous regions in different experimental groups. All data are representative of results from 3 independent experiments with 5 mice from each experimental group at each time point. All values are represented as means ± SD. Statistical analyses were done by ANOVA with Tukey’s post hoc analysis. In panels b and c, comparisons were done between the group receiving BCG, nanocurcumin, and H37Rv and all other experimental groups. * denotes a P value of ≤0.05. Experimental groups are (i) H37Rv, (ii) nanocurcumin plus H37Rv, (iii) BCG plus H37Rv, and (iv) BCG plus nanocurcumin and H37Rv.