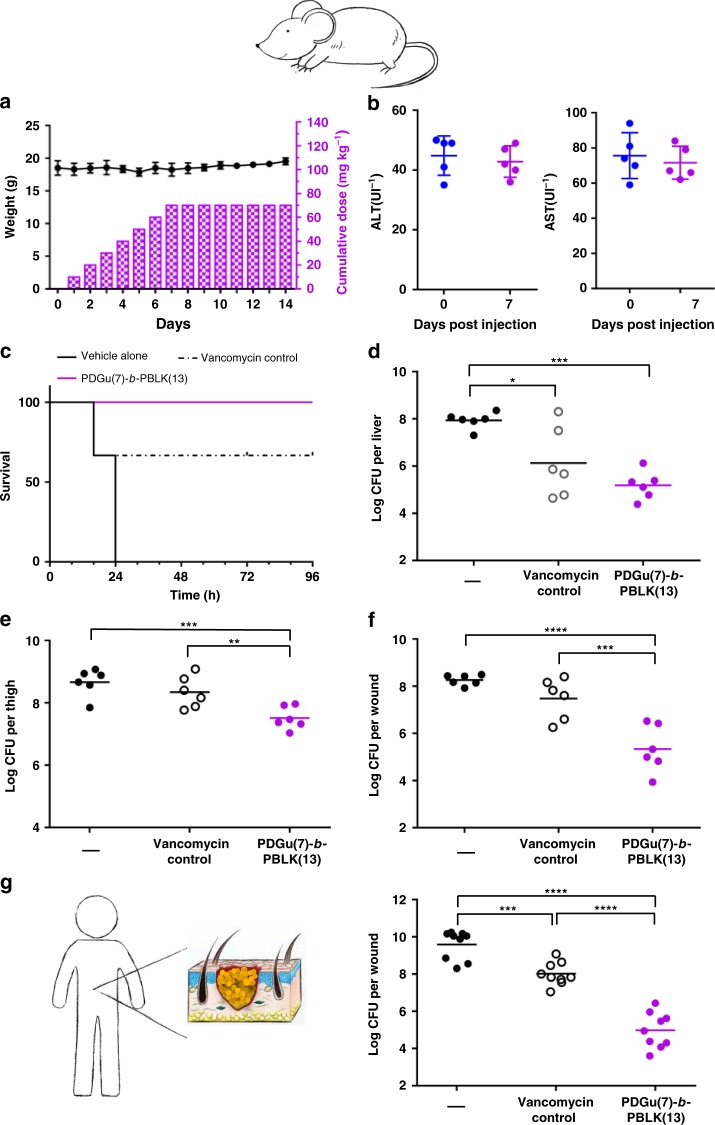
Fig. 6.
PDGu(7)-b-PBLK(13) is efficacious in vivo against MRSA USA300 with no toxicity. a, b In vivo repetitive toxicity of daily 10 mg kg−1 i.v. injection of PDGu(7)-b-PBLK(13) for 7 consecutive days. a Mice weight (left y-axis) and cumulative dosage (right y-axis) over 14 days. b ALT and AST biomarker changes at t = 0 and 7 days. Data are presented as mean ± standard deviation. c Survival% and d bacteria log reduction in liver in a systemic infection model. Vehicle alone (–), PDGu(7)-b-PBLK(13), or vancomycin control at 5 mg kg−1 were applied at a single dose, 2-h post infection. e In vivo antimicrobial activity of PDGu(7)-b-PBLK(13) against MRSA USA300 in a deep-seated neutropenic thigh infection model. First treatment was applied 24-h post infection at 20 mg kg−1, with a second dose at 20 mg kg−1 applied 3 h later. f In vivo antimicrobial activity of PDGu(7)-b-PBLK(13) against MRSA USA300 in an established murine excision wound model. Vehicle alone (–), PDGu(7)-b-PBLK(13), or vancomycin control at the same dosing (i.e. 2.5 mg kg−1) were applied six times over 2 days, starting 72-h post infection. g Ex vivo antimicrobial activity of PDGu(7)-b-PBLK(13) against MRSA USA300 in an established wounded human skin model. Vehicle alone (–), PDGu(7)-b-PBLK(13), or vancomycin control at 100 µg were applied three times with 3-h interval between treatments, starting 48 h post infection; **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 by one-way ANOVA followed by Dunnett test