Abstract
Aims:
The aim of the study is to evaluate the profile of peri-implant tissues in periodontally compromised patients.
Settings and Design:
In vivo – cross sectional study design.
Materials and Methods:
Fifty-eight implants were evaluated, clinically and radiographically, installed in seven individuals treated by the same team of professionals, during the years 1997 and 2005 in a private dental clinic in Vitória, ES, Brazil; that time of data collection, all implants were at least 10 years of functional loading. The variables related to the dental implants evaluated were: visible Plaque Index, Gingival Bleeding Index (GBI), probing pocket depth, bleeding on probing, and bone level, to relate them to the classification of dental implants.
Statistical Analysis Used:
The Chi-square and Kruskal–Wallis test were adopted.
Results:
The total of 58 implants were classified: 11 (18.9%) as healthy and 12 (20.7%) as clinically stable. The other 35 implants (60.4%) had some type of peri-implant inflammation, 20 of them (34.5%) were diagnosed with peri-implant mucositis and 15 (25.9%) with peri-implantitis. Among the variables studied, the results showed statistically significant differences for implant location (P = 0.001) and GBI (P = 0.03). Most of the maxillary implants (85.7%) were classified for some type of peri-implant disease. For the implants which resulted in Score 1 for GBI, most of them (75.0%) were also classified for some type of peri-implant disease.
Conclusions:
Dental implants placed in periodontally compromised patients may have high long-term survival rates. However, most implants were classified with some type of peri-implant inflammation.
Keywords: Implants, mucositis, peri-implantitis, periodontal diseases, risk factors
INTRODUCTION
In recent decades, dental implants in the replacement of missing teeth have become the treatment of choice for most patients and even professionals, and due to technical and scientific advances, this treatment presents high long-term survival rates.[1,2] Despite this, dental implants may present with inflammatory diseases classified as peri-implant mucositis, when an inflamed mucosa is observed with no signs of bone loss, or peri-implantitis, defined as the presence of inflammation in the mucosa, simultaneously with bone loss around the implant.[3]
Peri-implant mucositis may progress to peri-implantitis and even if the pathogenic mechanism was not yet clear, many similarities with periodontitis had already been recognized, such as the presence of known pathogens of periodontal disease.[4] The term peri-implantitis was first described in the study of Mombelli et al.[5] as an infectious disease. After that, a growing interest to define peri-implant inflammatory diseases has been observed. However, two decades after the first definition of peri-implantitis, most of these studies continued to present a diversity of criteria in the diagnosis of these diseases. This difference of criteria in the definition of peri-implant diseases becomes very clear when we compare the Roos-Jansåker et al.[6] study with the review of Zitzmann and Berglundh.[7] Roos-Jansåker et al.[6] observed 218 patients for 9–14 years, reporting that 16% of them had peri-implantitis. Zitzmann and Berglundh[7] reinterpreted these same results and stated that 55.6% of the patients had peri-implantitis. The difference observed in the results of these two studies was the criterion adopted to define peri-implantitis considered different numbers of threads without bone support, that is, in the study of Roos-Jansåker et al.,[6] the bone level (BL) should be apical to the third thread, whereas for Zitzmann and Berglundh,[7] the BL should be apical to the first thread of the implant. In both studies, it was considered bone loss should be associated with bleeding on probing (BoP).
Despite advances in the area, the systematic review by Derks and Tomasi[8] shows there are no clear diagnostic criteria for peri-implant mucositis or peri-implantitis in the scientific literature. The lack of diagnostic criteria used to describe the peri-implant diseases makes it difficult to compare results, and the studies present a great variability in the reports. Thus, in this review, the prevalence of peri-implant mucositis ranged from 19% to 65% and from 1% to 47% for peri-implantitis.
Peri-implant diseases are not evenly distributed among patients treated with dental implants, preferentially affect groups which patient profiles are at high risk for their establishment and development.[9] The clinical and microbiological similarity between periodontal disease and peri-implantitis gave rise to more research with dental implants installed in periodontally compromised patients. The possibility of transmission of periodontal pathogens to peri-implant sites in partially edentulous individuals with a history of periodontal disease could be considered a risk factor for the development of peri-implant diseases.[10] Although the history of periodontal disease presents as a risk factor, there is still no consensus regarding its influence on the long-term prognosis of implant therapy.[11]
The aim of this study was to evaluate the condition of peri-implant tissues, classifying implants for the presence or absence of peri-implant diseases in periodontally compromised patients after 10–18 years of loading.
MATERIALS AND METHODS
This study was realized in accordance with the Helsinki Declaration of human studies and the Resolution 466/12 of the National Health Council after approval of the Human Research Ethics Committee, under No. 733,536.
The sample selection was performed based on the evaluation of patient records, rehabilitated with dental implants, installed between 1997 and 2005, by the same team of professionals from a private clinic in the city of Vitória, ES, Brazil, following the same clinical protocol.
In the present study, individuals who had lost at least one tooth due to periodontal disease were diagnosed as periodontally compromised patients. Therefore, were recruited to evaluation: Periodontally compromised patient, partially edentulous with complete clinical documentation, rehabilitated with dental implant of external hexagon connection and screwed-retained single crown and/or multiple partial prostheses, placed adjacent to other implants or natural teeth, for a period of 10–18 years in functional loading, enrolled in a periodontal maintenance program. Were excluded of the study:
Individuals who had taken antibiotics or anti-inflammatory drugs within 2 months before the data collection
Individuals who did not sign the free and informed consent form
Smokers’ individuals
Implants with fractured prosthetic crowns
Individuals diagnosed with moderate-to-severe chronic periodontitis, that is, who had suppuration in some teeth, BoP in more than 30% of the subgingival sites, considering teeth and implants, or who had any teeth with pockets depth more than 5 mm
Individuals diagnosed with aggressive periodontitis, that is, they had a Plaque Index disproportionate to the marked loss of periodontal clinical attachment reaching mainly first molars and permanent incisors
Diabetic individuals.
First, a survey of the information in clinical and radiographic files of the individuals selected to record the following data was performed:
Personal information
Etiological reasons of tooth loss and the date of extraction
Date of implant placement
Type of prosthesis on the implant (single crown and/or multiple partial prostheses)
Implant location (anterior maxilla, posterior maxilla, anterior mandible, or posterior mandible)
Bone type (alveolar or bone graft)
Implant manufacturer
Implant length and
Implant diameter.
At the beginning of the clinical examination, the patients were informed about the proposal of the study, its risks and benefits, and asked to sign the informed consent term. An update was also made on the personal data of the patients and those related to general health, confirming those remained within the inclusion criteria.
Clinical examination
All patients underwent clinical examination, performed by a single calibrated professional, and not involved in the surgical and/or prosthetic treatment. To perform the intraexaminer calibration, before the beginning of the research, the selected professional was submitted to the Kappa test. The calibration was continued until the agreement was excellent (>0.75).
For all implants evaluated, the clinical parameters were recorded in a specific clinical file:
Visible Plaque Index (VPI) – dichotomous data were expressed, the presence (Score 1) or ab-sence (Score 0) was evaluated
Gingival Bleeding Index (GBI) – dichotomous data were expressed, the presence (Score 1) or absence (Score 0) was evaluated
Probing pocket depth (PPD) – measured in millimeters, as shown in Figure 1
BoP – dichotomous data.
Figure 1.
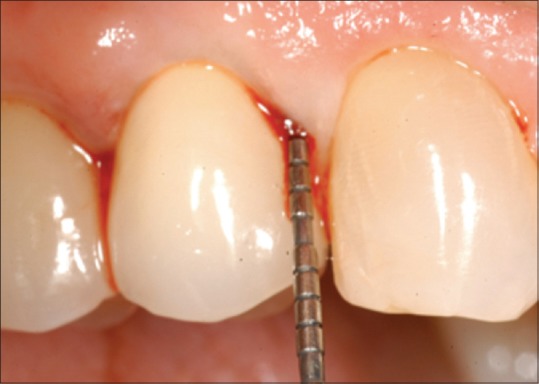
Peri-implant probing pocket depth, measured in millimeter, at the follow-up
All measurements were performed for each implant using a periodontal probe (PCPNU 15 Hu-Friedy Inc., Chicago, IL, USA).
Radiographic examination
For the evaluations of the marginal BL, digital intraoral periapical radiographic images of the 58 implants were obtained in a specialized radiological center. These periapical radiographs were obtained in Joint Photographic Experts Group format and evaluated by two calibrated professionals not involved in the surgical and/or prosthetic treatment. Intra- and inter-examiner calibrations were performed using the Kappa test and the results obtained a concordance >0.65. The radiographs were evaluated in the same environment to standardize the place where they would be evaluated by the professional. Thus, the BL in the mesial and distal of each implant was verified using the Windows Photo Viewer Program.
The BL radiographic parameter used was that indicated by Mir-Mari et al.,[12] which considers this dichotomous data and classifies it according to the number of threads of the implant without bone support, as shown in Figure 2. The thread pitch was identical for all implants evaluated, with approximately 0.6 mm. This parameter was measured on the mesial and distal surface of each implant to verify if the BL was apical to the second implant thread or not.
Figure 2.
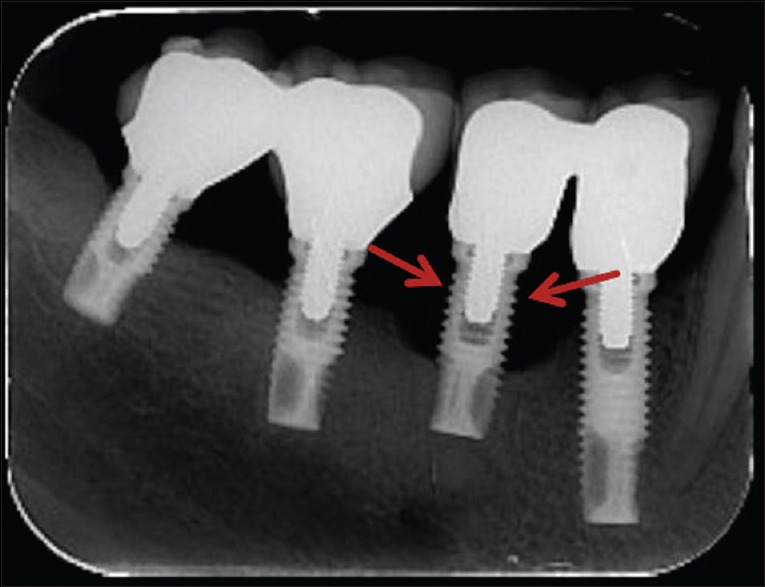
Digital intraoral periapical radiographic showing an implant with apical bone level to the second thread of the implant at the follow-up (red arrows point to the second thread)
Implant classification
After collection of clinical and radiographic data, each implant was classified as follows, as defined by Mir-Mari et al.:[12]
a. Health – BL <2 thread without BoP
b. Clinical stability – BL ≥2 thread without BoP
Inflammation
c. Peri-implant mucositis – BL <2 thread with BoP
d. Peri-implantitis – BL ≥2 thread with BoP or suppuration.
Statistical analyzes
In the present study, the implant was a sample unit. The classification of the implant's variable presents four distinct levels (health, clinical stability, peri-implant mucositis, and peri-implantitis). When tested with dichotomous variables, the Chi-square test was used and when the variable was also presented as a polychotomic, a contingency table was constructed for Chi-square application. In the case of numerical (quantitative) variables, a normality analysis was applied (assumption of several parametric tests), and according to the result, the Kruskal–Wallis test was adopted.
Statistical tests were performed in R-project 3.2.0 statistical software (R Core Team, Vienna, Austria). The level of significance established for the tests was 5%, which established a 95% confidence interval for the presented results, and the power of a statistical test was 80%.
RESULTS
Of the 58 implants evaluated, in seven individuals (3 males and 4 females), 21 were installed in male and 37 in female patients, aged between 63 and 80 years (mean 70.8 ± 1.32 years). Six of them had a college degree. The mean loading time for the implants was 13.4 ± 3.07 years, and each patient selected received, at least, five dental implants. The classification of 58 implants was as follows: 11 (18.9%) were classified as healthy and 12 (20.7%) as clinical stability. The other 35 implants (60.4%) were diagnosed with peri-implant disease, of which 20 (34.5%) had peri-implant mucositis and 15 (25.9%) had peri-implantitis, as shown in Table 1.
Table 1.
Demographic data and implant classification for the presence of peri-implant diseases
| Patient | Gender | Age | Number of implants | Health | Stability | Mucositis | Peri-implantitis |
|---|---|---|---|---|---|---|---|
| 1 | Male | 76 | 5 | 1 | 0 | 0 | 4 |
| 2 | Female | 68 | 12 | 3 | 3 | 2 | 4 |
| 3 | Male | 68 | 9 | 1 | 0 | 8 | 0 |
| 4 | Female | 80 | 7 | 3 | 0 | 2 | 2 |
| 5 | Female | 63 | 11 | 1 | 5 | 0 | 5 |
| 6 | Male | 75 | 7 | 0 | 0 | 7 | 0 |
| 7 | Female | 76 | 7 | 2 | 4 | 1 | 0 |
| Total (%) | 58 (100.0) | 11 (18.9) | 12 (20.7) | 20 (34.5) | 15 (25.9) | ||
Regarding the classification of implants for the presence or absence of peri-implant diseases, the variables loading time (P = 0.41), type of prosthesis (P = 0.62), type of bone (P = 0.07), implant manufacturer (P = 0.13), implant diameter (P = 0.18), implant length (P = 0.30), and VPI (P = 0.12) did not present a statistically significant difference. The variables location of the implant (P = 0.001) and the GBI (P = 0.03) presented a statistically significant difference (P = 0.001), as shown in Table 2.
Table 2.
Distribution of the different variables regarding the presence of peri-implant diseases
| Implant variable | n (%) | Peri-implant disease, n (%) | P |
|---|---|---|---|
| Number of implants | 58 (100) | 35 (60.4) | |
| Prothesis type | |||
| Unit | 11 (18.9) | 8 (72.7) | 0.62 |
| Multiple | 47 (81.1) | 27 (57.4) | |
| Implant location* | |||
| Maxilla | 28 (48.2) | 24 (85.7) | 0.001 |
| Mandible | 30 (51.8) | 11 (36.6) | |
| Bone type | |||
| Own | 52 (89.6) | 29 (55.7) | 0.07 |
| Bone grafting | 6 (10.4) | 6 (100) | |
| Manufactured | |||
| Steri-Oss | 10 (17.2) | 6 (60.0) | 0.13 |
| 3I | 29 (50.0) | 16 (55.1) | |
| Branemark | 16 (27.5) | 10 (62.5) | |
| Conexão | 3 (5.2) | 3 (100) | |
| Diameter (mm) | |||
| Narrow (<3.75) | 5 (8.6) | 4 (80.0) | 0.18 |
| Regular (3.75 to 4.1) | 37 (63.7) | 20 (54.0) | |
| Large (>4.1) | 16 (27.7) | 11 (68.7) | |
| Length (mm) | |||
| <10 | 8 (13.7) | 6 (75.0) | 0.30 |
| 10 to 12 | 24 (41.3) | 13 (54.1) | |
| >12 | 25 (43.1) | 16 (64.0) | |
| Gingival bleeding index* | |||
| Present | 32 (55.1) | 24 (75) | 0.03 |
| Absent | 26 (44.9) | 11 (42.3) |
*Statistically significant difference (P<0.05)
The mean of the PPD of the 58 implants was 5.1 ± 1.07 mm. The means of PPD found in the groups classified as the presence or absence of peri-implant disease did not present a statistically significant difference (P = 0.22), as shown in Table 3.
Table 3.
Data related to the implants classification regarding the average probing
| Implant classification | n | PPD (mean), mm | BL >2 threads (mesial or distal), n (%) | BoP sites, n (%) |
|---|---|---|---|---|
| Health | 11 | 4.5±0.73 | ||
| Clinical stability | 12 | 5.5±1.00 | 5 (41.6) | |
| Peri-implant diseases | 35 | 81 (57.0) | ||
| Mucositis | 20 | 5.2±0.79 | 44 (55.0) | |
| Peri-implantitis | 15 | 5.3±1.50 | 10 (66.6) | 37 (61.6) |
| Total | 58 | 5.1±1.07 | 15 (25.8) | 81 (34.9) |
PPD: Probing pocket depth, BL: Bone level, BoP: Bleeding on probing
DISCUSSION
The present study evaluated a homogeneous group of partially edentulous individuals, composed of seven individuals with 58 implants with at least 10 years of function. Considering the bacterial composition of the biofilm formed in implants is similar as adjacent teeth, this study did not include in the sample total edentulous patients, since the subgingival microbiota found in teeth of partially edentulous individuals could present as a reservoir of periodontal pathogens in individuals with a history of periodontal diseases.[13]
In relation to the number of individuals, the sample of the present study is smaller than other cross-sectional studies.[14,15,16] However, studies by Mir-Mari et al.[12] and Rokn et al.[1] include implants in function for <5 years, considered the insufficient time to evaluate the development of peri-implant diseases. According to Karoussis et al.,[13] Sgolastra et al.,[10] and Zangrando et al.,[14] a follow-up of at least 5 years is required to access the information of the peri-implant tissues, thus ensuring that the information observed is a consequence of the interaction of the microbiota in the peri-implant sites. In addition, unlike the present study, these cross-sectional studies do not present information regarding the inclusion or not, in their samples, patients with smoking habits, diabetes, or a history of periodontal disease. It is worth emphasizing that these variables could influence the development of peri-implant disease.[15] In the present study, the dental implant was used as the sample unit, due to the sample size. Most studies investigating peri-implant diseases also show their results in this way.[16,17,18,19]
The classification used to define the presence of peri-implant diseases was the same one used in the cross-sectional study of Mir-Mari et al.[12] These authors found the results: 21.4% of the implants were diagnosed for some type of peri-implant disease, of which 15.4% had peri-implant mucositis and 6.0% had peri-implantitis. The present study showed a higher number of implants with peri-implantitis when compared with the study of Mir-Mari et al.[12] in addition to other studies, such as Roos-Jansåker et al.[6] who found 6.6% of implants classified with peri-implantitis and Rokn et al.[1] who found 8.8% of 13 implants classified with peri-implantitis.. This difference may have occurred because the present study presents a sample considered a risk group for the development of peri-implant diseases. The study of Marrone et al.[15] was observed a prevalence of 23% for peri-implantitis, a result closer to that found in the present study. For the authors, this difference occurred in the group of total edentulous patients, with a history of periodontal disease. The authors believe that periodontal pathogens may persist for a long time in the oral cavity, even in edentulous individuals with a history of periodontal disease and when they lose their teeth they prone to neglect oral hygiene measures due to lack of motivation, favoring the inadequate control of plaque, which may also influence the development of peri-implant diseases.
In the study of Karoussis et al.,[13] the patients were classified in the same way as in the present study, that is, those who had lost at least one dental element due to periodontal diseases and compared them with periodontally healthy patients. The result found in the group of periodontally compromised patients was 28.6% of implants diagnosed with peri-implantitis, a result like that of the present study, which found 25.9%. The results found for periodontally healthy individuals were 5.8% of implants diagnosed as such. Similarly, in a study, Marrone et al.[15] were evaluated individuals with a history of periodontal disease, and the result found was 28% of implants diagnosed for peri-implantitis. There is evidence to support the hypothesis the history of periodontal disease seems to predispose the patient to a greater risk of biological complications, as the peri-implant diseases.[16] Thus, these individuals treated with dental implants could present peri-implant surfaces colonized by these pathogens within a few weeks after implant placement.[17] This process possibly occurs through bacterial translocation from periodontal to the peri-implant tissues.[18]
In this study, were obtained the measurement of a BL according to the situation of the marginal bone in relation to the implant threads at the time of the evaluation, like that found in other cross-sectional studies.[15,16,17,18] Peri-implant bone changes can be induced by remodeling after loading or due to a subsequent infection.[19,20] However, when the BL is apical to the second thread or 2 mm apical to the implant platform, probably related to the peri-implantitis, and this bone loss may be associated with an active peri-implantitis or a previous peri-implantitis, successfully treated.[21] The bone destruction caused by peri-implantitis is characteristic, presenting as circumferential craters around the implant. Even though the implant is losing support bone due to peri-implantitis, the apical portion maintains the implant stabilized, causing bone loss to proceed without notable signs of implant mobility until the osseointegration failed.[22]
Corroborating with Mir-Mari et al.,[12] none of the two studies was a statistically significant difference for PPD between the groups of implants diagnosed. The results of the two studies show the mean of PPD could not differentiate implants that presented peri-implant diseases, those that diagnosed as healthy or clinical stability. Other studies also did not present a statistical difference in the mean of PPD in implants diagnosed for some diseases when compared to those without signs of peri-implant inflammation.[22,23,24] Although the PPD is an excellent index for evaluating the periodontal health of teeth, this index presents differently when used to evaluate dental implants due to the difference in the arrangement of the found in the periodontium and peri-implant fibers.[23] However, although PPD does not permit the diagnosis of peri-implant diseases, are the alterations in his results, such a progressive increase of pocket probing depth, which will suggest the presence of inflammation at peri-implant sites.[24] Similarly, the mean of PPD of the present study was not able to differentiate implants that presented BL located apical to the second thread, of those who did not present such bone loss, as in the work of Mir-Mari et al.[12] The studies of Lee et al.[16] and Karoussis et al.[13] also showed no correlation between PPD and BL, showing that implants with less deep pockets (<4 mm) could present higher levels of bone loss than implants with deeper pockets (>5 mm). For some authors, it is possible to estimate the extent of the peri-implant lesions and the bone destruction that is closer to reality through radiographic examinations, when compared to the peri-implant pockets.[25]
The present study showed a statistically significant difference for the location of the implant variable, with a higher number of implants classified for some type of peri-implant disease, in those placements in the maxilla when compared to those in the mandible. Individuals who have lost some dental element due to periodontal disease may present reduced amounts of bone tissue for treatment with dental implants. When rehabilitating a patient with a dental implant in posterior areas of the maxilla, that present reduction of bone tissue, the treatment can present a lower survival rate, since it is a region that presents a bone tissue with poor quantity and quality, that is, predominantly marrow bone.[26] This poor bone in the posterior maxillary areas may still lead to the need for short implants and consequently, the need to increase the size of the prosthetic crown. This disproportion of length between implant and prosthetic crown could decrease the survival rates and accentuate the biological complications of the implants. When tooth extractions occur in the anterior areas of the maxilla and the etiologic reason is periodontal disease, bone defects may happen vestibular dehiscence defects, that is, very thin or even absent. Therefore, bone grafting may be necessary for the treatment of implants in these areas, since the vestibular bony wall and postextractions are reabsorbed in a few weeks and the use of grafts would also favor biological complications of implants.[27]
In this study, another variable that presented a statistically significant difference was the GBI. In the study of Mir-Mari et al.,[12] implants diagnosed for some type of peri-implant disease present slightly higher GBI than implants without any inflammation. In the study of Karoussis et al.,[13] the authors reported higher GBI on implants in periodontally compromised patients, that is, in the group that presented a greater number of implants with inflammatory diseases. Similarly, Mengel and Flores-de-Jacoby[23] observed that implants with higher GBI showed a greater peri-implant bone loss. However, in the studies of Karoussis et al.,[13] Mengel and Flores-de-Jacoby,[23] and Mir-Mari et al.,[12] the authors did not report whether there was a statistically significant difference in the results regarding the GBI. Shibli et al.[26] evaluated implants diagnosed with peri-implantitis and healthy implants. Implants diagnosed with peri-implantitis presented higher GBI and greater marginal bone loss when compared to healthy implants, and these two variables showed a statistically significant difference. According to Mombelli and Décaillet,[24] BoP related to histopathological changes in the inflamed tissues of the peri-implant sites, such as increased vascularization and the presence of ulcerations in the sulcular epithelium. A higher peri-implant vascularization indicates the presence of many cells and little collagen, thus greater inflammation, and the manifestation of these inflammations directly related to the profile of the microbiota present and the immune response of the host to these microorganisms.[27] More studies are needed with clearer criteria for the classification and diagnosis of peri-implant diseases to increase the knowledge of the etiopathogenesis and better the understanding of the risk factors for the development of these diseases.
CONCLUSIONS
It can be concluded that it is possible to rehabilitate periodontally compromised patients with dental implants. The study showed that the definition of peri-implantitis presents variability among the studies, and despite the high survival rates, it has to be realized that individuals with a history of periodontal disease are more susceptible to peri-implant diseases.
Financial support and sponsorship
The Foundation for Research Funding in Espírito Santo supported this research (grant 007/2014, 0459/2015).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rokn A, Aslroosta H, Akbari S, Najafi H, Zayeri F, Hashemi K. Prevalence of peri-implantitis in patients not participating in well-designed supportive periodontal treatments: A cross-sectional study. Clin Oral Implants Res. 2017;28:314–9. doi: 10.1111/clr.12800. [DOI] [PubMed] [Google Scholar]
- 2.Jain JK, Sethuraman R, Chauhan S, Javiya P, Srivastava S, Patel R, et al. Retention failures in cement- and screw-retained fixed restorations on dental implants in partially edentulous arches: A systematic review with meta-analysis. J Indian Prosthodont Soc. 2018;18:201–11. doi: 10.4103/jips.jips_25_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang NP, Berglundh T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now? – Consensus of the Seventh European workshop on periodontology. J Clin Periodontol. 2011;38(Suppl 11):178–81. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 4.Cho-Yan Lee J, Mattheos N, Nixon KC, Ivanovski S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin Oral Implants Res. 2012;23:325–33. doi: 10.1111/j.1600-0501.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 5.Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. The microbiota associated with successful or failing Osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Roos-Jansåker AM, Lindahl C, Renvert H, Renvert S. Nine – To fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J Clin Periodontol. 2006;33:290–5. doi: 10.1111/j.1600-051X.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 7.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 8.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42:158–71. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 9.Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177–89. doi: 10.1034/j.1600-0757.2002.280107.x. [DOI] [PubMed] [Google Scholar]
- 10.Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Periodontitis, implant loss and peri-implantitis. A meta-analysis. Clin Oral Implants Res. 2015;26:e8–16. doi: 10.1111/clr.12319. [DOI] [PubMed] [Google Scholar]
- 11.Anitua E, Piñas L, Begoña L, Orive G. Long-term retrospective evaluation of short implants in the posterior areas: Clinical results after 10-12 years. J Clin Periodontol. 2014;41:404–11. doi: 10.1111/jcpe.12222. [DOI] [PubMed] [Google Scholar]
- 12.Mir-Mari J, Mir-Orfila P, Figueiredo R, Valmaseda-Castellón E, Gay-Escoda C. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J Clin Periodontol. 2012;39:490–4. doi: 10.1111/j.1600-051X.2012.01872.x. [DOI] [PubMed] [Google Scholar]
- 13.Karoussis IK, Salvi GE, Heitz-Mayfield LJ, Brägger U, Hämmerle CH, Lang NP. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI dental implant system. Clin Oral Implants Res. 2003;14:329–39. doi: 10.1034/j.1600-0501.000.00934.x. [DOI] [PubMed] [Google Scholar]
- 14.Zangrando MS, Damante CA, Sant’Ana AC, Rubo de Rezende ML, Greghi SL, Chambrone L. Long-term evaluation of periodontal parameters and implant outcomes in periodontally compromised patients: A systematic review. J Periodontol. 2015;86:201–21. doi: 10.1902/jop.2014.140390. [DOI] [PubMed] [Google Scholar]
- 15.Marrone A, Lasserre J, Bercy P, Brecx MC. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin Oral Implants Res. 2013;24:934–40. doi: 10.1111/j.1600-0501.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Kim HM, Kim CS, Choi SH, Chai JK, Jung UW. Long-term retrospective study of narrow implants for fixed dental prostheses. Clin Oral Implants Res. 2013;24:847–52. doi: 10.1111/j.1600-0501.2012.02472.x. [DOI] [PubMed] [Google Scholar]
- 17.Buser D, Chappuis V, Bornstein MM, Wittneben JG, Frei M, Belser UC. Long-term stability of contour augmentation with early implant placement following single tooth extraction in the esthetic zone: A prospective, cross-sectional study in 41 patients with a 5- to 9-year follow-up. J Periodontol. 2013;84:1517–27. doi: 10.1902/jop.2013.120635. [DOI] [PubMed] [Google Scholar]
- 18.Coli P, Christiaens V, Sennerby L, Bruyn H. Reliability of periodontal diagnostic tools for monitoring peri-implant health and disease. Periodontol 2000. 2017;73:203–17. doi: 10.1111/prd.12162. [DOI] [PubMed] [Google Scholar]
- 19.De Boever AL, Quirynen M, Coucke W, Theuniers G, De Boever JA. Clinical and radiographic study of implant treatment outcome in periodontally susceptible and non-susceptible patients: A prospective long-term study. Clin Oral Implants Res. 2009;20:1341–50. doi: 10.1111/j.1600-0501.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 20.Tandan A, Upadhyaya V, Raghuvanshi M. Comparative evaluation of the influence of immediate versus delayed loading 34 protocols of dental implants: A radiographic and clinical study. J Indian Prosthodont Soc. 2018;18:131–8. doi: 10.4103/jips.jips_127_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. Peri-implantitis. Periodontol 2000. 2010;53:167–81. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- 22.Matarasso S, Rasperini G, Iorio Siciliano V, Salvi GE, Lang NP, Aglietta M. A 10-year retrospective analysis of radiographic bone-level changes of implants supporting single-unit crowns in periodontally compromised vs. periodontally healthy patients. Clin Oral Implants Res. 2010;21:898–903. doi: 10.1111/j.1600-0501.2010.01945.x. [DOI] [PubMed] [Google Scholar]
- 23.Mengel R, Flores-de-Jacoby L. Implants in patients treated for generalized aggressive and chronic periodontitis: A 3-year prospective longitudinal study. J Periodontol. 2005;76:534–43. doi: 10.1902/jop.2005.76.4.534. [DOI] [PubMed] [Google Scholar]
- 24.Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol. 2011;38(Suppl 11):203–13. doi: 10.1111/j.1600-051X.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- 25.Polizzi G, Grunder U, Goené R, Hatano N, Henry P, Jackson WJ, et al. Immediate and delayed implant placement into extraction sockets: A 5-year report. Clin Implant Dent Relat Res. 2000;2:93–9. doi: 10.1111/j.1708-8208.2000.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 26.Shibli JA, Melo L, Ferrari DS, Figueiredo LC, Faveri M, Feres M. Composition of supra – And subgingival biofilm of subjects with healthy and diseased implants. Clin Oral Implant Res. 2008;19:975–82. doi: 10.1111/j.1600-0501.2008.01566.x. [DOI] [PubMed] [Google Scholar]
- 27.Belibasakis GN. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch Oral Biol. 2014;59:66–72. doi: 10.1016/j.archoralbio.2013.09.013. [DOI] [PubMed] [Google Scholar]


