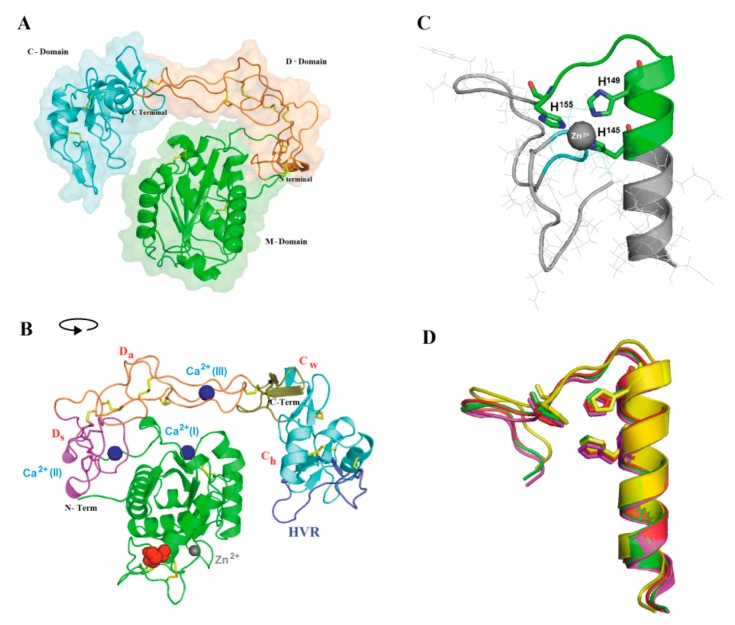
Figure 3.
Theoretical Atroxlysin-III 3D model. (A) A homology model of Atr-III generated with Modeller 9.13 using crystal structures of other P-III SVMPs as templates. The metalloproteinase (M), disintegrin-like (D) and Cysteine-rich (C) domains are indicated by green, orange, and cyan colors, respectively. Elements of secondary structure α-helices, β-structures, N- and C-terminal ends are presented. Disulfide bonds are in yellow sticks. (B) Principal motifs of Atr-III according to Igarashi et al. [34]. The M-domain, Ds, Da, Cw, and Ch segments and the hyper-variable-region (HVR) are shown in green, magenta, orange, olive, cyan, and blue, respectively. Zinc and calcium ions are represented as grey and blue spheres, respectively. N-glycosylation motif is indicated in red spheres. (C) Details of the zinc binding site of Atr-III where the positioning of the three histidine residues are shown. (D) Superposition of the zinc-binding residues of Atr-III with the P-III SVMPs are shown in sticks and labeled. B. jararaca (red), C. atrox (magenta), and A. acutus (yellow). For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.