Abstract
The formation of carcinogenic bromate ions is a constraint when ozone is used for the remediation of water containing brominated organic materials. With its strong oxidizing ability, ozone rapidly transforms bromide in aqueous media to bromate, through a series of reactions involving hydroxyl radicals. Several strategies, such as limiting the ozone concentration, maintaining pH < 6, or the use of ammonia or hydrogen peroxide were explored to minimize bromate generation. However, most of the above strategies had a negative effect on the ozonation efficiency. The advanced oxidation processes, using catalysts together with ozone, have proven to be a promising technology for the degradation of pollutants in wastewater, but very few studies have been conducted to find ways to minimize bromate formation during this approach. The proposed article, therefore, presents a comprehensive review on recent advances in bromate reduction in water by catalytic ozonation and proposes reaction mechanisms associated with the catalytic process. The main aim is to highlight any gaps in the reported studies, thus creating a platform for future research and a quest to find environment friendly and efficacious catalysts for minimizing bromate formation in aqueous media during ozonation of brominated organic compounds.
Keywords: bromate minimization, catalytic ozonation, metal oxides, bromide
1. Introduction
The need to reduce environmental pollution is currently receiving urgent attention around the world. The rapid increase in the human population, coupled with growing demands from industrial and other sectors, has triggered the large-scale usage of diverse non-biodegradable chemicals, leading to extensive pollution of water systems. Since these polluted waters pose a serious threat to the environment, ongoing research is conducted to explore cost effective treatment methodologies for the removal of varied toxic chemicals from the water systems. An alternative to chlorination and adsorption agents for water purification is ozonation, which is becoming a useful methodology for improving the quality of water. The use of ozone has proven to be excellent for microorganism destruction and biological contaminant removal from water [1], but is not effective for degrading recalcitrant organic pollutants in water. The presence of bromide in polluted waters poses a serious problem during ozonation. Bromide is rapidly oxidized to toxic bromate during ozone treatment. Bromide is usually present in low concentrations of between 104 and 106 ppb in wastewaters and approximately 67 103 ppb in seawater [2]. Relatively low amounts were found in rainwater, ranging from 0 to 110 ppb [3], but in groundwater, between 10 and 2 × 103 ppb were detected [4]. Higher bromide concentrations have been reported in waters and soil samples near oceans [5]. Mining and leaded petrol [6], fertilizers and insecticides are considered major sources of bromine contamination of the environment and aquatic systems [7]. Bromide was also found in many treated water facilities ranging from 3000 to 10,000 ppb [8,9]. If bromide levels as low as 20 ppm are present in water during ozonation, the potential exists for bromate formation to occur through a combination of ozone and hydroxyl radical reactions [1]. Bromate is a known human carcinogen [10,11,12] and its maximum allowable limit in drinking water is set at 10 ppb or lower [13]. Therefore, it is crucial to minimize or prevent its formation in drinking water.
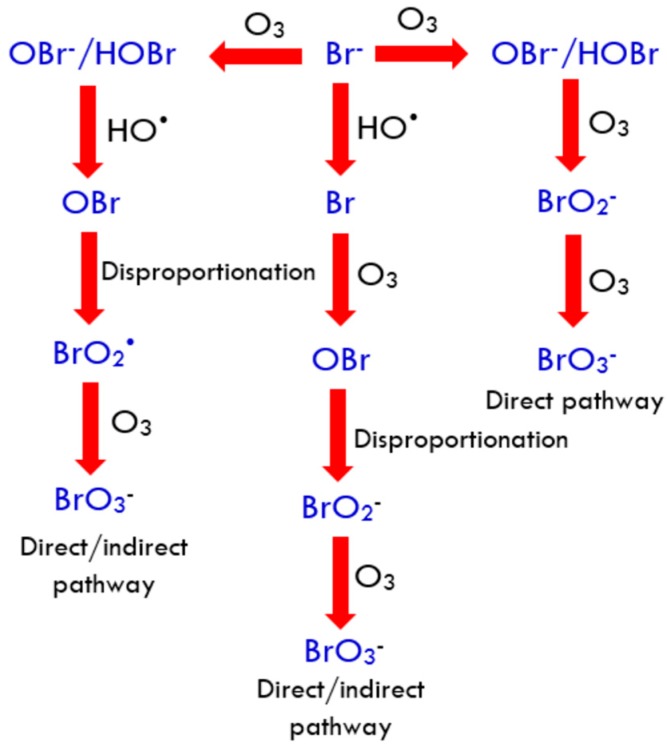
In aqueous systems, ozone oxidizes bromide to bromate via three different pathways [14]. The dominance of a particular pathway is dependent on the amount of bromide, organic carbon and pH of the substrate solution. As illustrated in Figure 1, the first pathway (direct pathway) is initiated by the reaction of bromide ion with molecular ozone to form . The is further oxidized by dissolved to and finally to . The second pathway (direct/indirect pathway) is facilitated by the molecular ozone, resulting in the formation of . However, in this route the formed is oxidized by radicals to a series of highly reactive oxygenated radicals. Further ozonation produces ions. According to Richardson et al. [15], this pathway is favoured if solution pH and alkalinity of the water is high. In the third pathway, the radicals interact with bromide ions resulting in the generation of radicals, which is disproportionate to bromite ions. The bromite ions are then oxidized by molecular ozone to produce bromate ions.
Figure 1.
Bromate formation pathways [14].
The use of suitable heterogeneous catalysts has proven to be beneficial to enhance the efficiency of the ozonation process and minimize the generation of toxic by-products [16]. Studies have shown that hydroxyl radicals generated during ozonation in the presence of metal oxides could increase bromate formation [17]. This review presents a comprehensive assessment on recent advances on bromate reduction in water by heterogeneous catalytic ozonation.
2. Bromate Minimization Strategies
The following mechanism was proposed by von Gunten and Hoigne’ for the conversion of to during ozonation [18]:
They concluded that the direct oxidative conversion of to was mainly controlled by molecular ozone, while further oxidation of to radicals was influenced by radicals. The unstable radicals disproportionate to . The dissolved ozone in the water then rapidly oxidizes to [1]. Limited studies have been conducted to establish the effects of catalytic ozonation on bromate formation. The most recent studies are discussed below.
2.1. MCM-48, CeO2 and Cex-MCM-48
Li et al. [19] reported on catalytic ozonation of bromide containing waters with MCM-48, CeO2 and combined mesoporous sieve Cex-MCM-48 (cerium combined with MCM-48) with various Si/Ce molar ratios (Ce30/66/100/200-MCM-48). All catalysts were able to considerably impede formation in comparison to ozonation alone. After 30 min of ozone treatment, the inhibition efficiencies of MCM-48 and CeO2 were 78.6% and 63.9%, respectively. When MCM-48 was doped with Ce, a marked improvement in minimization was observed. When the Ce content was increased from x = 200 to x = 66, yield decreased, giving a maximum inhibition efficiency of 91% after 30 min of ozonation. However, an additional increase of Ce to x = 30, resulted in an increase in concentration and an inhibition efficiency of 78%. Their explanation for this trend was that doping MCM-48 with Ce resulted in the generation of more surface hydroxyl groups, which successively enhanced decomposition of O3 on the active sites of the catalyst surface. However, doping beyond x = 66 blocked the active sites, leading to a destruction of the mesoporous structure of MCM-48, hence leading to poor catalyst activity.
Li et al. [19] proposed a bromate reduction pathway for Ce66-MCM-48 with the aid of bromine mass balance studies. Their results revealed that Ce66-MCM-48 did not adsorb and , the main bromine-containing species present in the water solution. The amounts of both and in Ce66-MCM-48 ozonation were expressively lower, relative to ozone in absence of catalyst, while the amount of was much higher in Ce66-MCM-48 ozonation. As the bromide oxidation is primarily controlled by , Ce66-MCM-48 ozonation tends to prevent production by limiting the influence of direct oxidation. The results have shown that decomposed faster with Ce66-MCM-48 (82% decomposition after 5 min), in comparison to ozonation alone (53% decomposition in the first 5 min). Since a lower amount of dissolved exists in Ce66-MCM-48 ozonation, the consecutive oxidation reactions from are all inhibited. The generated secondary oxidant, reacts with some bromine containing species, organic micropollutants, or combine to form . The results showed that concentration steadily increases during ozonation alone, reaching a maximum value of 0.6 M after 20 min. With ozonation in the presence of Ce66-MCM-48, a higher concentration was detected, but it remained constant (1.5–1.7 M) for the entire 20 min. Another bromate inhibition mechanism involved electron transfer reactions between and on Ce66-MCM-48 surface. These reactions lead to the inhibition of to , thus resulting in lower formation. The surface ions underwent oxidation by and to form [20] according to the following pathway:
| (1) |
| (2) |
Ce3+ also reacts with to form [21]:
| (3) |
An alternative pathway produces from aqueous decomposition
| (4) |
| (5) |
| (6) |
| (7) |
Ce3+ is regenerated by , which converts to [21]:
| (8) |
2.2. α-FeOOH, α-Fe2O3, γ-FeOOH and CeO2
T. Zang et al. [22] investigated the effect of a number of metal oxides, such as , , and on bromate production during ozone treatment of bromide in water. The catalytic reactions with produced more relative to ozonation alone, whereas the reactions with , and minimized bromate formation. However, was most active in reducing bromate production. They determined simultaneously the concentrations of and for uncatalysed ozonation and CeO2 catalysed ozonation. They found that the amounts in catalytic ozonation was lower with ozone treatment alone before 15 min, and remained similar thereafter. The amount in catalytic ozonation was always significantly higher in comparison to ozone treatment alone. According to von Gunten [1], is an essential intermediary for production during ozonation, therefore, its accumulation in catalytic ozonation suggests that considerably inhibits the conversion of to .
The formation of was detected in both ozonation alone and ozonation with . The results showed that the amount of with was poorer compared to single ozonation. Studies have shown that the surface of can initiate the decomposition generating oxygen in water [23]. Therefore, the lesser amount in catalytic ozonation can be attributed to its concurrent disintegration on the surface of. One study mentioned that low amounts of hydrogen peroxide can promote formation, arising from hydroxyl radical formation from the interaction of with [1], and other studies discussed that hydrogen peroxide at high amounts ( molar ratio >1:2) is likely to reduce to , hence minimizing formation [17,18,24]. According to Zang et al. [22], the enhanced minimization in catalytic ozonation is primarily due to the lower amounts. Since catalytic ozonation produced a lower amount of than single ozonation, the amount is expected to be moderately lower, hence resulting in a lower oxidation rate of to . Furthermore, can be reduced to by , which is a temporary reductive state of surface in catalytic decomposition of [25]. Thus, an additional pathway for minimization is the reduction of to on the surface. Both reduction routes require the involvement of surface active sites.
It has been reported that ions, when combined with metal oxides, have a strong attraction for their surface sites [26]. Zang et al. [22], therefore, added various concentrations of to the bromide containing solutions to ascertain its affinity for surface active sites, and the impact on . They found that the difference in bromate formation between ozonation alone and catalytic ozonation decreased as amounts increased from 0 to 5 mM. The diminishing effectiveness of to minimize formation is ascribed to surface co-ordination, thus indicating that surface sites account for most of the minimization during catalytic ozonation.
2.3. Nano-Metal Oxides, SnO2 and TiO2
Wu et al. [27] conducted simulation studies to investigate the influence of nano-metal oxides, and on bromate generation in pure water during ozone treatment. Their results showed that ozonation in the presence of nano-metal oxides ( and ) as catalysts, minimized generation to a greater extent, compared to single ozonation. However, nano- was most effective in inhibiting formation. The experimental results showed that the concentrations of residual O3 and were significantly lesser in nano-TiO2 catalysed ozonation relative to uncatalysed ozonation and nano-SnO2 ozonation, indicating that catalytic ozonation with nano-TiO2 decomposes more O3 to radicals. The lower ozone concentration results in lower, hence minimizing formation. Furthermore, radicals can rapidly combine to generate , which can reduce to [28,29]. The presence of humic acid influenced bromate generation. Increasing the humic acid concentration from 0 to 3.0 ppm resulted in a decrease in bromate formation. Humic acid reacts readily with O3 and hydroxyl radicals, which also reacts with and [16,30]. Therefore, a lower concentration of leads to lesser bromate formation [22].
2.4. Mn Incorporated MCM-41
Xue et al. [31] employed mesoporous Mn incorporated MCM-41 to hinder bromate production during catalytic ozonation of waters containing bromide. A comparison of the three temperature ramping rates (0.5 K min−1, 1 K min−1 and 2 K min−1) during calcination of MnX-MCM-41 (X = 40, 80, 100 and 120, the molar ratio of Si/Mn), revealed that Mn100-MCM-41 with ramping rate of 1 K min−1 showed superior surface characteristics and the greatest bromate inhibition efficiency. A 96.7% inhibition efficiency was achieved after 60 min when compared to ozonation alone. XPS data revealed that Mn100-MCM-41 (1 K min−1) has more oxygen vacancies, which has tendency to adsorb and dissociate to surface active species [32]. Ozone readily reacts with these surface-active species, resulting in less ozone exposure for oxidation to , hence minimizing bromate formation. The higher fraction of and in Mn-MCM-41 enhanced bromate inhibition efficiency.
Xue et al. revealed that the concentration of during Mn100-MCM-41 ozonation was lower than single ozonation. They explained that Mn100-MCM-41 adsorbs and dissociates to form surface active species. Ozone then readily reacts with these surface-active species, hence leading to low ozone exposure for oxidation . Furthermore, hydrogen peroxide was detected in both uncatalysed and Mn100-MCM-41 catalysed ozonation. The concentration of increased steadily in Mn100-MCM-41 ozonation, but decreased in uncatalysed ozonation, signifying that more reactive oxygen species [32] is formed in the presence of Mn100-MCM-41. These species are capable of consuming and preventing bromate formation. To verify the role of hydroxyl radicals, TBA (a potential radical scavenger) was introduced in both single ozonation and Mn100-MCM-41 ozonation. The bromate yield decreased for both processes, thus confirming that was primarily responsible for production. In ozonation alone, the decrease in bromate yield is mainly attributed to the decrease in hydroxyl radicals. In Mn100-MCM-41/O3 process, the decreased bromate yield is due to the decrease in both hydroxyl radicals and residual ozone. A similar phenomenon was evident with Fe-Cu-MCM-41 [33].
2.5. Fe-MCM-41, Cu-MCM-41 and Fe-Cu-MCM-41
Chen et al. [33] showed that ozonation with Fe-MCM-41, Cu-MCM-41 and Fe-Cu-MCM-41 catalysts considerably reduced formation. The inhibition activity and bromate yield were as follows: Cu-MCM-41 (28.8 ppb) ≈ Fe-MCM-41 (31.5 ppb) > Fe-Cu-MCM-41 (124.5 ppb) > O3 (432.5 ppb). They attributed the bromate reduction to ozone decomposition by the catalysts, resulting in a reduced amount of ozone for bromate generation [19]. The higher bromate yield in Fe-Cu-MCM-41/O3 than in Fe-MCM-41/O3 and Cu-MCM-41/O3 systems, is due to more presence in the Fe-Cu-MCM-41/O3 system. The presence of both the redox couples, and on the catalyst surface (confirmed by XPS analysis) further accelerated ozone decomposition into radicals. As illustrated in Figure 2, bromate is produced through both the direct and indirect oxidation of by [34].
Figure 2.
Formation route for bromate.
After the addition of the catalyst, more ozone is consumed, resulting in a hindrance of the direct oxidation of to by ozone (a key intermediate reaction for bromate generation), and additional oxidation of to [19]. The superior efficiency of Fe-Cu-MCM-41, causes an abundance of hydroxyl radicals. A greater concentration results in an impediment of pathway 1, thus resulting in a higher bromate build-up [35].
The addition of t-butanol (TBA) to the substrate solution, generated less bromate in both single ozonation and ozonation with Fe-Cu-MCM-41. As reported, the bromate formation requires the presence of both ozone and hydroxyl radicals [36]. Bromide is first oxidized by ozone directly to . Thereafter, the is oxidized by to . Thus, in single ozonation, since the radicals are scavenged by TBA, bromate formation is primarily due to molecular ozone. In the Fe-Cu-MCM-41/O3 process, the ozone concentration in the water significantly decreases due to the surface reactions, and the generated radicals are also scavenged by TBA. Both actions result in the suppression of the bromate formation pathway, hence, lowering bromate yield.
Bromate production was also inhibited in both ozonation alone and Fe-Cu-MCM-41 catalytic ozonation with the addition of . Bromide yields were found to increase with an increase in dosage. As proposed by Huang, accelerates the generation of , which reduces to , hence constraining generation [37].
2.6. Fe–Al LDH Supported on Mesoporous Al2O3
Nie et al. [38] prepared Fe–Al layered double hydroxides (Fe-Al LDH, the molar ratio of : = 1:10) supported on mesoporous and showed its effectiveness to minimize bromate formation. The concentration rapidly increased during the uncatalysed ozonation reaching 20 ppb after 60 min of ozone treatment. However, ozonation with Fe-Al LDH/Al2O3 completely inhibited formation. Furthermore, even when the initial concentration and ozone dose were increased, the yield after 60 min of catalytic ozonation stayed below the allowable limit of 10 ppb.
Fe-Al LDH/Al2O3 in the presence of a mixture of phenazone (PZ) and only, revealed that approximately 45% of was adsorbed on Fe-Al LDH/Al2O3 and 18% of was generated. They ascribed the reduction to formed during Fe-Al LDH/Al2O3 preparation, which was confirmed by XPS analysis [39]. However, 82% of was converted to during Fe-Al LDH/Al2O3 ozonation of the PZ/ mixture. The reduction of to increased with the ozone dose and concentration. In contrast, the PZ/O3 system could not reduce to . Furthermore, when phosphate was added to the Fe-Al LDH/Al2O3/O3 system, reduction was completely suppressed. The presence of phosphate permanently blocked the active surface sites of the catalyst, resulting in the replacement of surface hydroxyl groups and the formation of complexes with within the catalyst, thereby decreasing catalytic activity [40,41]. The adsorption of and the interaction of O3 with Fe-Al LDH/Al2O3 was suppressed, therefore, poor reduction is expected. Further investigations indicated that reduction to by surface is responsible for complete inhibition of formation. The needed for reduction is generated from surface reactions occurring on Fe-Al LDH/Al2O3. The - intermediate complex on the catalyst surface undergoes electron transfer reactions to produce . Furthermore, the reaction of with forms . The results also revealed that bromate reduction was favoured in the presence of different organic pollutants during catalytic ozonation. The amount of surface , confirmed by XPS analysis, on Fe-Al LDH/Al2O3 varied for different organic pollutants, suggesting that the structure of the organic pollutant had an impact on the reduction of .
2.7. Mesoporous Alumina Supported MnOx
Nie et al. [42] investigated the reduction pathway of generation during ozonation of 2,4-dichlorophenoxyacetic acid (2,4-D) with mesoporous alumina supported MnOx (MnOx/Al2O3) suspension. The ozonation of 2,4-D in the presence of bromide resulted in a rapid increase in bromate yield. The degradation of 2,4-D was significantly suppressed, while the efficiency of TOC removal decreased significantly from 25.7% to 7%. The catalytic ozonation with MnOx/Al2O3 significantly inhibited formation, however, the presence of did not influence 2,4-D degradation.
In agreement with other studies, was found to be the main essential intermediate for formation [18]. During both the uncatalysed and catalysed ozonation, was rapidly generated. However, generation was significantly supressed with MnOx/Al2O3 in comparison to single ozonation. The trend in the data suggested that different bromine transformation mechanisms existed in the two processes. Bromate reduction occurred over MnOx/Al2O3 with ozone and 2,4-D, while a rapid increase in yield was observed. The results confirmed that was reduced to on the surface of MnOx/Al2O3 during ozonation. Electron transfer reactions occurred during the adsorption and decomposition processes on the surface of the catalyst [43,44,45]. The UV–Vis absorption spectrum of MnOx showed the existence of Mn in different oxidation states, namely , and [46]. Therefore, is responsible for promoting to eliminate organic pollutants and also assist in inhibiting formation. The proposed reactions on MnOx/Al2O3 in the presence of ozone occurs as follows [42]:
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
Reaction (14) proposes the generation of in both uncatalysed and catalytic ozonation. The results showed that concentration was remarkably lower in uncatalysed ozonation than in MnOx/Al2O3 catalytic ozonation. This trend suggests that in catalytic ozonation, reaction (14) is suppressed, since more is used up by reactions (10) and (11), hence leading to increased generation of . This confirmed that the presence of different oxidation states of manganese is responsible for controlling generation.
2.8. Cex Zrx-1O2 Mixed Oxides
Yang et al. [47] prepared mixed oxides (x = 0.16, 0.50, 0.75, 0.9) and to study reduction during ozonation of containing filtered water. The results indicated that catalytic ozonation with and minimized bromate formation better than ozonation alone. They concluded that the mixed oxides and effectively suppressed the oxidation of by and radicals. Furthermore, the mixed oxide displayed the best catalytic activity for minimization, with 53% of formation being reduced after 20 min of ozonation. The adsorption of and on catalyst surface were not detected, since anions have no affinity for the neutral or negatively charged oxide surface. Furthermore, the catalyst material exhibited good stability, since no leaching of metal ions were detected during the ozonation process.
To confirm the role of and radicals in inhibition, p-chlorobenzoic acid (pCBA), a scavenger was introduced to monitor radicals. HPLC analysis revealed that pCBA concentration decreased rapidly with ozone treatment time, and its concentration was considerably lower in ozonation than in single ozonation. This indicates that mixed oxide significantly promoted the decomposition of to radicals during the catalytic ozonation process. Their results also showed that formation and decomposition was extremely rapid during the first 5 min of ozonation, further confirming that radicals play a major role during formation. The organic compounds in water favours organic/ reactions more than / reactions, since the rate of reaction for oxidative degradation of organic compounds by radicals is faster than that for oxidizing by radicals [48]. Since the radicals facilitate the efficient degradation of organic substituents, therefore, the suppression of the oxidation of is favoured, leading to the minimization of yield.
2.9. TiO2
Parrino et al. [49] investigated simultaneous ozonation and photocatalysis for purifying wastewater containing formic acid/4-nitrophenol and bromide ions. The initial ozonation experiments performed on formate and bromide ions in the presence and absence of TiO2, showed similar degradation rates, suggesting that reactions occurring on the TiO2 surface did not contribute to the degradation of the target compounds [50]. It was also observed that the oxidation of formate was not affected by the presence of bromide ion and the oxidation of bromide to bromate occurred only after the consumption of formate ions. Bromide ions reacted with hydroxyl radicals generated during photocatalysis, according to the following reaction scheme:
| (15) |
| (16) |
| (17) |
Lastly, the photoelectrons generated on the photocatalyst surface reduced the hypobromite species to bromide.
| (18) |
As illustrated, these pathways eventually lead to the recovery of bromide ion, Equation (18). Furthermore, if solution pH is in the range 6–8, a secondary pathway facilitates the conversion of hypobromous acid to bromide. The generated HOBr, as shown in Equation (16), primarily exists in its protonated form, and H2O2 generated during the photocatalytic reaction, acts as a scavenger for hypobromite, by reducing it to bromide [51].
From this outcome, they concluded that bromate generation can be prevented by interrupting the ozone treatment as soon as the oxidation of the organic species is almost complete. Furthermore, reducing bromate is also a more practical way to minimize its accumulation, and as per the previous reports, photocatalysis alone is efficient to convert bromate to bromide [51]. When 4-nitrophenol was substituted in the place of the formate ion, the formation of bromate, took place once again only after the disappearance of 4-nitrophenol, and was found to be faster than with formate ion. This implies that the type of organic contaminant in the water plays a decisive role in the amount of bromate formed.
2.10. β-FeOOH/Al2O3
Nie et al. [52] investigated bromate formation during the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) in containing water under uncatalysed and catalysed ozonation conditions. In uncatalysed ozonation, bromate yield increased rapidly to a maximum value of 21.5 ppb, but in . ozonation, formation was completely inhibited.
Furthermore, the experimental data showed that about 68% of was adsorbed on the surface of during 2,4-D degradation, and with the addition of ozone, was completely converted into within 180 min. In ozonation without 2,4-D, was not reduced to. However, reduction was found to only occur with selected organic contaminants.
Results also showed that no Fe2+ was formed when was present in water alone, however, a small amount of surface Fe2+ was observed when in water was ozonated. A further increase in surface Fe2+ was noticed when water in the presence of and 2,4-D was ozonated. The quantity of surface Fe2+ decreased rapidly when was introduced, signifying that Fe2+ was responsible for conversion to [39,53]. The Fe2+ generated on arises from the reaction of Fe3+ with , and the complexation of surface Fe3+ with the oxy-functional groups (-OH, -COOH). The organic pollutants or their oxygenated intermediates improves the reaction of Fe3+ with , hence resulting in more surface Fe2+, which causes a higher reduction rate.
2.11. Fe-Cu-MCM-41
Chen et al. [29] investigated the formation of bromate in the presence of Fe-Cu-MCM-41 during ozonation of /Diclofenac containing water. They found that Fe-Cu-MCM-41 decreased the concentration of dissolved ozone, hence diminishing the direct reaction of O3 with. Ozonation of water containing only bromide ions, produced 276 ppb bromate, but in the presence of Fe-Cu-MCM-41 the bromate yield decreased to 151 ppb. Bromide in the presence of Diclofenac (DCF), saw a significant drop in bromate formation for both O3 alone and Fe-Cu-MCM-41/O3. During the initial treatment process, is oxidized to and then to under the action of O3 and radicals. The presence of DCF and its intermediates influences formation by competing with and for O3 and radicals, thus inhibiting bromate formation. Also, the degradation of DCF decreases the solution pH to acidic, and bromate formation is not favoured in acidic medium [35].
2.12. Perovskite-Type Oxides, LaFeO3 and LaCoO3
Y. Zhang et al. [54] synthesized two perovskite-type oxides, LaFeO3 and LaCoO3, and examined their capacity to degrade benzotriazole (BZA) and minimize formation in water during ozonation. The ozonation of an aqueous mixture of BZA and generated the most amount of . The bromate yield increased sharply for the first 20 min of ozonation and then showed a decreasing trend up to 120 min. The bromate yield decreased significantly after the addition of catalyst, especially during the first 30 min of ozonation, but the conversion of was faster with LaCoO3 compared with LaFeO3. The concentration of was found to be higher in LaCoO3 ozonation than with LaFeO3, which explains its superior minimization ability. The production of resulted in the generation of H2O2, which also contributed to the reduction of to .
Y. Zhang et al. [54] further illustrated the reaction mechanism of LaFeO3 and LaCoO3 facilitated ozonation of benzotriazole (BZA) and minimization. They concluded that LaFeO3 did not catalytically promote molecular ozone decomposition to reactive oxygen species (ROS), which is needed for BZA degradation, but instead rapidly reduced. The reaction of over LaFeO3, suggested that was used up in the presence of LaFeO3 and the consumed was not used to produce radicals. The in [Fe-]S more easily reduces to .
On the other hand, LaCoO3 promoted the decomposition of ozone to ROS, which facilitated faster degradation of BZA and oxidation of to . Therefore, concentration was lower in the presence of LaCoO3 than in ozonation alone. LaCoO3 accelerated the decomposition of BZA to . The reduced directly to form more . The cyclic reaction of Co3+/Co2+ also promoted BZA degradation and inhibition of reduction.
2.13. HZSM-5 Zeolites
T. Zhang et al. [55] studied the influence of H+-form high silica ZSM-5 (HZSM-5) zeolites with different Si/Al molar ratios (i.e., 25–300) on bromate formation. Their results showed that bromate yield increased with time in a single ozonation, O3/HZSM-5 and O3/CeO2. The bromate concentration in O3/HZSM-5 was significantly lower than in single ozonation and in O3/CeO2. The HZSM-5 with Si/Al ratios of 300 and 25 showed superior capacity for bromate minimization and reduced approximately 58% bromate formation potential after 20 min of ozone treatment, while CeO2 only reduced 22%. Further studies on HZSM-5 (Si/Al = 300) showed that its high efficiency for bromate minimization is related to its affinity to adsorb , a major intermediate in bromate formation [1]. The results have shown that HZSM-5 had no affinity to adsorb of , and HOBr, therefore no direct electron transfer reaction is expected on HZSM-5. However, the majority of was rapidly adsorbed onto HZSM-5 within 0.5 min. They then concluded that the specific adsorption of on the HZSM-5 prevents the oxidation of to in water. Their results also detected the presence of in both single ozonation and ozonation with HZSM-5. Considerably higher yields of were detected in single ozonation than in O3/HZSM-5 process, and the HZSM-5 neither adsorbed nor decomposed in water. The lower concentration in O3/HZSM-5 leads to lower bromate yields.
2.14. FeOX/CoOX
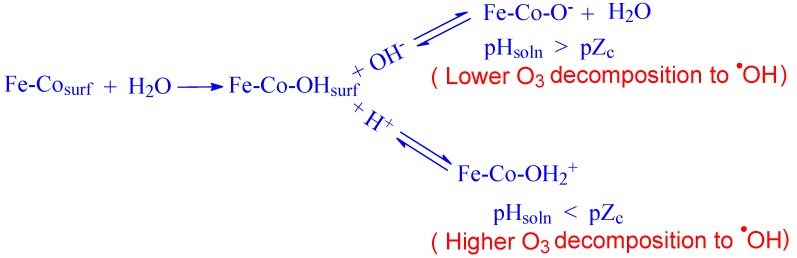
Gounden et al. [56], conducted a study on the degradation of hazardous halohydrin, 2,3-dibromopropan-1-ol (2,3-DBP) in water by ozonation alone and ozonation with Co loaded on Fe prepared by co-precipitation (Co-ppt) and a simple physical mixing method (Mixed). Their results showed that debromination of 2,3-DBP produced large quantities of and ions. The Fe:Co (Mixed) catalyst was found to be more effective in suppressing the generation of bromate than the Fe:Co (Co-ppt) catalyst. The presence of Fe:Co (Mixed) lowered the solution pH from 6.8 to 5.7, which was an ideal condition for inhibiting bromate formation. The reaction pathway for conversion of to was described in the presence of Fe-Co (Mixed) catalyst. Firstly, since pH of the initial solution (5.7), is higher than the pZc value (5.1) of the Fe-Co (Mixed) catalyst, its surface can comprise mostly of negative active sites (Scheme 1). These sites repel the negatively charged bromide ions, thus preventing electron transfer reactions on the catalyst surface, resulting in a lower bromate yield.
Scheme 1.
Reaction pathway for formation of protonated/deprotonated Fe-Co surface in water.
Secondly, since the pH of the initial solution is much lower than the pKa (8.8) of the HOBr/OBr−system, an equilibrium shift occurs to the left, thus favoring a higher yield of HOBr and lower . As ozone is more reactive towards HOBr than , a decrease in bromate yield is anticipated (Scheme 2).
Scheme 2.
Pathway for inhibition/formation of bromate in catalytic ozonation.
A similar pattern of bromate formation, as illustrated in Scheme 3, was observed when 2,4,6-Tribromophenol (2,4,6-TBP) was ozonated with Fe-Co metal oxides. Using Fe-Co (Mixed) catalyst, only 5% of the available bromide was oxidized to bromate, whereas with Fe-Co (Co-ppt), 39% of bromide was converted.
Scheme 3.
Reaction pathways for bromate formation during Fe-Co catalysed ozonation and degradation of 2,4,6-TBP in water.
3. Factors Affecting Bromate Minimization
3.1. Effect of Initial Solution pH
Previous studies have shown that lowering of pH to below 7, preceding ozonation results in a decrease in bromate formation [57]. A decrease of one pH-unit results in 50–63% reduction in formation [58]. This decrease has been attributed to two factors: (i) At pH < 7, oxidized bromide is likely to primarily be found as hypobromous acid , resulting in limited amounts of hypobromite available for reaction with ozone [18,59]:
As the solution pH is increased, the concentration of increases, hence promoting production, since is more reactive with ozone than HOBr [1]. The main oxidant for bromate formation in natural water is the hydroxyl radical. At a lower pH, the conversion of molecular ozone to hydroxyl radicals is low, therefore, the amount of bromate formed through the hydroxyl radical pathway is limited. At lower pH, the ratio of hydroxyl radical to ozone tends to be lower than at higher pH. The lowering of pH can also be problematic because it can result in poor or incomplete degradation of organic substrates, which can lead to the formation of various hazardous brominated organic compounds. Furthermore, for high alkalinity wastewaters, the lowering of pH is not economically feasible.
Li et al. [19] studies confirmed that bromate formation increased significantly in ozonation alone as pH was increased from 6.3 to 9.5. This can be due to fact that in alkaline medium (i) shifts the acid/base equilibria of 8.8) towards , which reacts readily with both and [1], and (ii) decomposes to radicals, which enhances formation. Their Ce66-MCM-48/O3 system minimized formation and was also pH dependant. For pH range of 7.6–8.6, a higher minimization efficiency of 87–91% was attained by Ce66-MCM-48 after 10 min of ozonation. With a decrease in pH to 6.3, the inhibition efficiency decreased to 76%. When the pH was increased to 9.5, the minimization efficiency of Ce66-MCM-48 reduced to 82%. At high pH, is the major species. It reacts rapidly with both and to form significant amounts of .
The experiments conducted by T. Zhang et al. [22] at controlled pH revealed that yield increased rapidly in both single ozonation and in the system as the pH was increased from 5.5 to 8.9. An 84% reduction in yield was achieved at pH 6.2. They attributed the catalytic activity and reduction to the surface charge of and intermediary speciation, which are pH dependent. When the pH of the solution is close to the of (6.6), its surface is not charged. If solution pH is below the of its surface becomes positively charged, due to protonation of its surface hydroxyl sites by water. This condition increases the proportion of , hence minimizing formation. If solution pH is above the of its surface becomes negatively charged due to deprotonation of surface hydroxyl sites, thus continuously increasing the quantity of , which favours the formation of bromate ion.
Wu et al. [27] monitored formation at different pH values during single ozonation and ozonation with nano. Their results indicated that ozonation with nano favoured the formation of as solution pH increased initially from 6.0 to 7.9. They also concluded that at high pH, rapid ozone decomposition is favoured, hence increasing production of hydroxyl radicals, resulting in higher formation. A higher proportion of is present at pH 7.9, which would also promote formation. The increasing pH led to a slight decrease in formation rate from 73.75% to 71.32%, displaying a reduced activity for nano.
Xue et al. [31] observed that the initial solution pH had a significant influence on bromate formation during ozonation in the presence of Mn100-MCM-41(1 K min−1). The inhibition efficiencies for bromate formation were 96.7%, 83.4% and 68.2% at pH 6.5, 7.5 and 9.5 respectively. The increase in bromate formation with pH, is influenced by the equilibrium of and the stability of ozone in aqueous media. The increasing pH favours the formation of more ions, which readily decomposes to form radicals, therefore, accelerating bromate formation. In acidic conditions, ozone is stable and more is present, therefore, bromate formation is suppressed [60].
Chen et al. [33] observed that by increasing the initial solution pH from 3.0 to 9.0 increased bromate formation for both uncatalysed and Fe-Cu-MCM-41 catalysed ozonation, however, for the entire pH range Fe-Cu-MCM-41/O3 process generated lower bromate yield. As the pH increased to 9.0, bromate steadily accumulated, reaching 913 ppb in single ozonation and 335 ppb in Fe-Cu-MCM-41 ozonation. At the acidic condition, is favoured (pH < pKa), and since is more stable, fewer radicals are formed. As predominantly reacts with , the oxidation pathway 2 in Figure 2 is suppressed and a reduced amount of bromate is formed. Under basic conditions, the equilibrium shifts towards , which is highly reactive towards both and , resulting in accelerated bromate production [35].
Zhang et al. studied the influence of pH on bromate formation for the O3/HZSM-5 system [55]. In ozonation alone, it was observed that as solution pH increased from 6.6 to 9.3, the bromate yield increased rapidly from 4.9 ppb to 27 ppb. In catalytic ozonation with HZSM-5, the bromate yield increased more steadily from 2.8 ppb to 9.4 ppb. They attributed the drop in bromate formation to the adsorption of on HZSM-5 at different pH levels. Considering the equilibrium constant of 10-9 for , the fraction of in at pH 8.0 and pH 9.3 is approximately 14% and 76%, respectively. This would mean that higher amounts of can be adsorbed on HZSM-5 at pH 9.3 than at pH 8.0, so would the bromate reduction efficiency. However, their results have shown that the percent reduction in bromate formation increased only by 7.6%, when the solution pH was raised from 8.0 to 9.3. Since is more reactive towards ozone than HOBr, and the reaction rate is approximately two times that of [1]. Therefore, the increase in pH leads to a substantial increase in bromate yield in single ozonation. In HZSM-5 ozonation, O3 and compete with HZSM-5 for , thus resulting in lower bromate formation at higher pH.
Kishimoto and Nakamura [61] concluded from their studies that hydroxyl radicals are more crucial than molecular ozone in bromate production. They demonstrated that in ozonation alone, yield increased as concentration decreased at neutral pH in the absence of 4-chlorobenzoic acid (4-CBA). However, yields considerably decreased compared to removal at acidic pH and in the presence of 4-CBA. Although acidic pH decreased generation, it limited the oxidation capacity of ozone for successful 4-chlorobenzoic acid degradation. Therefore, the acidification during ozonation is favorable for minimization, but it has the disadvantage of affecting the removal efficiency of organic pollutants from water.
3.2. Effect of Initial Bromide Concentration
Several studies have shown that the presence of small quantities of bromide ion can result in the generation of significant amounts of bromate ion during single ozonation. Bromate ion yield increased as bromide ion concentration increased. A few studies were conducted to investigate the influence of initial bromide concentration on the bromate formation during catalytic ozonation.
Wu et al. [27] examined formation for various initial concentrations during single ozonation and ozonation with nano. The data indicated that in single ozonation yield increased rapidly as a function of initial concentration, however in ozonation with nano the yield was significantly lower. When initial concentration increased from 0.4 ppm to 1.2 ppm, the reduction rate of decreased from 67.22% to 47.11%, suggesting that the activity of nano is severely inhibited with an increase in initial concentration.
The experiments conducted by T Zhang et al. [22] to study the influence of initial bromide concentration on bromate production showed that in single ozonation yield increased rapidly from 0.5 ppm to 2 ppm, as the concentration of bromide ion increased. In catalysed ozonation, formation was significantly suppressed for concentrations 1.0 ppm, however, for concentrations 1.0 ppm, yield started to increase rapidly. The yield in catalysed ozonation was always lower than that obtained with uncatalysed ozonation.
3.3. Effect of Ozone Dosage
Sufficient availability of ozone showed an increase in the bromate ion formation, until all bromide ion was converted to bromate ion [58]. von Gunten and Hoigne [18] have introduced a standard measure for the ozone concentration (C) as a function of reaction time (t), which is defined as the Ct value (mg/L·min) for ozone exposure. An increase in the quantity of ozone improves the Ct value during ozone treatment of water. Wu et al. [27] demonstrated that yield kept on increasing as ozone concentration was increased in both single ozonation and nano ozonation, that is, for all experiments formation increased linearly as the ‘Ct value increased. When ozone dosage was increased from 2.22 ppm to 4.62 ppm, an improvement in the reduction rate from 62.94% to 75.66% was observed. The formation rates in single ozonation were found to be much higher than in catalytic ozonation, however, no explanation was given for this trend.
Zhang et al. [55] showed that bromate yield increased rapidly from 7.8 ppb to 95 ppb in single ozonation as the ozone concentration was increased from 0.38 ppm to 1.16 ppm. In catalytic ozonation with HZSM-5, bromate yield increased much slower (from 4.3 ppb to 21 ppb) for the same increase in ozone dose. HZSM-5 may have depleted the concentrations of ozone and/or intermediate species, which are needed for bromate formation.
3.4. Influence of Temperature Changes
The increasing temperature generally increases bromate ion production in water during ozonation. The effects of temperature are due to the following facts: (i) Ozone decomposition into radicals is favoured at higher temperatures; (ii) an increase in temperature enhances the reaction rate and (iii) the pKa of the system is temperature dependent.
The experimental data showing the influence of solution temperature on bromate minimization efficiency indicated that in the temperature range of 15℃ to 30℃ Ce66-MCM-48 catalytic ozonation showed nearly the same minimization efficiency as that of single ozonation [19]. This temperature-independent feature of Ce66-MCM-48 is advantageous for water treatment by ozonation.
The influence of solution temperature on formation showed that, in single ozonation, the yield increased moderately when the temperature was increased from 5 °C to 15 °C, and increased more sharply when raised from 15 °C to 25 °C. The generation of in ozonation was found to be similar to single ozonation, however much less was produced in ozonation [22].
3.5. Influence of Catalyst Dosage
Generally, the bromate yield increases as a function of catalyst dose. For example, bromate production with increasing nano dosage (0 to 200 ppm) investigated by Wu et al. [27] showed that when nano dose was increased from 0 to 100 ppm, the reduction rate increased from 0% to 72.59%. However, when nano dose increased from 100 to 200 ppm, the reduction rate only went up to 74.27%. The nanoparticles have extremely high surface area, therefore, increasing nano dosage would result in more active catalytic sites for surface reactions. However, in aqueous solution, ozone concentrations are limited, hence the marginal increase in reduction rate.
4. Conclusions and Recommendations
The literature indicates that catalytic ozonation using appropriate catalyst materials is a better solution for bromate minimization than uncatalysed ozonation. However, there is still a need for more efficient and practically applicable catalysts to be explored for complete elimination of bromate formation during ozonation. All catalysts reported were able to significantly minimize formation in comparison to ozonation alone, however, only few were able to minimize bromate formation below the 5 ppb limit. The following bromate inhibition strategies/mechanisms during catalytic ozonation of bromide containing waters were proposed:
Increasing the number of hydroxyl groups on the catalyst surface resulted in enhanced ozone decomposition to radicals, thus limiting the contribution of direct for the sequential oxidation of . The formation of excess is beneficial for removal of organic pollutants from the water.
Redox reactions on the catalyst surface causes inhibition of and in some cases reduction of to , thus limiting bromate formation. The lesser concentration leads to lesser .
The generation of hydrogen peroxide was detected in most catalytic ozonation systems, but was found to be lower than in ozonation alone. The lesser means lesser radicals, therefore, the oxidation rate of to to is diminished. Contrary to this, some authors observed an increase in , which they attributed to the reactive oxygen species, which are capable of consuming . Further work on the relationship between generation and bromate inhibition is therefore needed.
The presence of phosphate and humic acid had a tendency to limit bromate formation, however, high levels of phosphate and humic acid can result in poor water quality.
The limited studies on photocatalytic ozonation of bromide containing waters showed that the concentration of hypobromite species can be minimized by the photoelectrons generated on the photocatalyst surface, thus contributing to bromate reduction.
Bromate reduction was enhanced in the presence of certain organic compounds, due to electron transfer reactions on the catalyst surface.
Some catalysts have an affinity to adsorb critical intermediate species () needed for bromate formation.
Mixed metal oxides were found to effectively minimize bromate formation by simply lowering the initial solution pH to more acidic levels.
Acknowledgments
The authors would like to thank Mangosuthu University of Technology and University of Kwa-Zulu Natal for the facilities and technical assistance given for successful completion of this work.
Author Contributions
For this review article, while all the required literature material was collected and draft compiled by A.N.G., the final manuscript was prepared and edited by S.B.J.
Funding
No funding is received for the preparation of this review article.
Conflicts of Interest
The authors declare no conflict of interest.
References and Note
- 1.von Gunten U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003;37:1469–1487. doi: 10.1016/S0043-1354(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 2.Heeb M.B., Criquet J., Zimmermann-Steffens S.G., von Gunten U. Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds—A critical review. Water Res. 2014;48:15–42. doi: 10.1016/j.watres.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Neal C., Neal M., Hughes S., Wickham H., Hill L., Harman S. Bromine and bromide in rainfall, cloud, stream and groundwater in the Plynlimon area of mid-Wales. Hydrol. Earth Syst. Sci. 2007;11:301–312. doi: 10.5194/hess-11-301-2007. [DOI] [Google Scholar]
- 4.Flury M., Papritz A. Bromide in the natural environment: Occurrence and toxicity. J. Environ. Qual. 1993;22:747–758. doi: 10.2134/jeq1993.00472425002200040017x. [DOI] [Google Scholar]
- 5.Yuita K. Iodine, bromine and chlorine contents in soils and plants of Japan. Soil Sci. Plant Nutr. 1983;29:403–428. doi: 10.1080/00380768.1983.10434645. [DOI] [Google Scholar]
- 6.Kittel, H. Substances Usable in Substitution of 2-nitropropane and 1,2 Dibromoethane (Ersatzstoffe für 2-nitropropan und 1,2 dibromoethan). Schriftenreihe Gefährliche Arbeitsstoffe, 1983, (No. 11. Dortmund. Wirtschaftsverlag NW, Bremerhaven.).
- 7.Bowen H.J.M. Environmental Chemistry of the Elements. Academic Press; London, UK: 1979. [Google Scholar]
- 8.Krasner S.W., McGuire M.J., Jacangelo J.G., Patania N.L., Reagan K.M., Aieta E.M. Occurrence of DBP’s in US drinking water. J. Am. Water Works Assoc. 1989;81:41–53. doi: 10.1002/j.1551-8833.1989.tb03258.x. [DOI] [Google Scholar]
- 9.Survey on Bromide in Drinking Water and Impacts on DBP Formation. American Water Works Research Foundation; Denver, CO, USA: 1994. [Google Scholar]
- 10.Legube B., Parinet B., Berne F., Croue J.-P. Bromate Surveys in French Drinking Waterworks. Ozone-Sci. Eng. 2002;24:293–304. doi: 10.1080/01919510208901620. [DOI] [Google Scholar]
- 11.Kruithof J.C., Kamp P.C., Martijn B.J. UV/H2O2 Treatment: A Practical Solution for Organic Contaminant Control and Primary Disinfection. Ozone-Sci. Eng. 2007;29:273–280. doi: 10.1080/01919510701459311. [DOI] [Google Scholar]
- 12.Kurokawa Y., Maekawa A., Takahashi M., Hayashi Y. Toxicity and carcinogenicity of potassium bromate-a new renal carcinogen. Environ. Health Persp. 1990;87:309–335. doi: 10.1289/ehp.9087309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Primary Drinking Water Regulations . Stage 2 Disinfectants and Disinfection By-Products Rule. Volume 71. US Environmental Protection Agency; Washington, DC, USA: 2006. pp. 387–493. [Google Scholar]
- 14.Song R., Amy G.L., Westerhoff P., Minear R. Bromate minimization during ozonation. J. Am. Water Works Assoc. 1997;89:69–78. doi: 10.1002/j.1551-8833.1997.tb08243.x. [DOI] [Google Scholar]
- 15.Richardson L., Burton D.T., Helz G.R. Residual Oxidant Decay and Bromate Formation in Chlorinated and Ozonated Sea-Water. Water Res. 1981;15:1067. doi: 10.1016/0043-1354(81)90074-9. [DOI] [Google Scholar]
- 16.Molnar J.J., Agbaba J.R., Dalmacija B.D., Klasnja M.T., Dalmacija M.B., Kragulj M.M. A Comparative Study of the Effects of Ozonation and TiO2-catalyzed Ozonation on the Selected Chlorine Disinfection By-product Precursor Content and Structure. Sci. Total Environ. 2012;425:169–175. doi: 10.1016/j.scitotenv.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 17.von Gunten U., Bruchet A., Costentin E. Bromate formation in advanced oxidation processes. J. Am. Water Works Assoc. 1996;88:53–65. doi: 10.1002/j.1551-8833.1996.tb06571.x. [DOI] [Google Scholar]
- 18.von Gunten U., Hoigne J. Bromate Formation during Ozonization of Bromide-Containing Waters: Interaction of Ozone and Hydroxyl Radical Reactions. Environ. Sci. Technol. 1994;28:1234–1242. doi: 10.1021/es00056a009. [DOI] [PubMed] [Google Scholar]
- 19.Li W., Lu X., Xu K., Qu J., Qiang Z. Cerium incorporated MCM-48 (Ce-MCM-48) as a catalyst to inhibit bromate formation during ozonation of bromide-containing water: Efficacy and mechanism. Water Res. 2015;86:2–8. doi: 10.1016/j.watres.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Pelle K., Wittmann M., Lovrics K., Noszticzius Z. Mechanistic Investigations on the Belousov−Zhabotinsky Reaction with Oxalic Acid Substrate. 2. Measuring and Modeling the Oxalic Acid−Bromine Chain Reaction and Simulating the Complete Oscillatory System. J. Phys. Chem. A. 2004;108:7554–7562. doi: 10.1021/jp047472a. [DOI] [PubMed] [Google Scholar]
- 21.Heckert E.G., Seal S., Self W.T. Fenton-Like Reaction Catalyzed by the Rare Earth Inner Transition Metal Cerium. Environ. Sci. Technol. 2008;42:5014–5019. doi: 10.1021/es8001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., Chen W., Ma J., Qiang Z. Minimizing bromate formation with cerium dioxide during ozonation of bromide-containing water. Water Res. 2008;42:3651–3658. doi: 10.1016/j.watres.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Hiroki A., LaVerne J.A. Decomposition of hydrogen peroxide at water-ceramic oxide interfaces. J. Phys. Chem. B. 2005;109:3364–3370. doi: 10.1021/jp046405d. [DOI] [PubMed] [Google Scholar]
- 24.Acero J.L., Haderlein S.H., Schmidt T., Suter M.J.F., von Gunten U. MTBE oxidation by conventional ozonation and the combination ozone/hydrogen peroxide: Efficiency of the processes and bromate formation. Environ. Sci. Technol. 2001;35:4252–4259. doi: 10.1021/es010044n. [DOI] [PubMed] [Google Scholar]
- 25.Aubry J.M. Search for singlet oxygen in the decomposition of hydrogen peroxide by mineral compounds in aqueous solutions. J. Am. Chem. Soc. 1985;107:5844–5849. doi: 10.1021/ja00307a002. [DOI] [Google Scholar]
- 26.Peak D., Ford R.G., Sparks D.L. An in situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite. J. Colloid Interface Sci. 1999;218:289–299. doi: 10.1006/jcis.1999.6405. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y., Wu C., Wang Y., Hu C. Inhibition of Nano-Metal Oxides on Bromate Formation during Ozonation Process. Ozone-Sci. Eng. 2014;36:549–559. doi: 10.1080/01919512.2014.904735. [DOI] [Google Scholar]
- 28.Wen G., Pan Z.-H., Ma J., Liu Z.-Q., Zhao L., Li J.-J. Reuse of sewage sludge as a catalyst in ozonation—Efficiency for the removal of oxalic acid and the control of bromate formation. J. Hazard. Mater. 2012;239–240:381–388. doi: 10.1016/j.jhazmat.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Chen W., Zhang Z., Li X., Wu D., Xue Y., Li L. Reducing DBP’s formation in chlorination of Br-containing Diclofenac via Fe-Cu-MCM-41/O3 peroxidation: Efficiency, characterization DBP’s precursors and mechanism. J. Taiwan Inst. Chem. Eng. 2018;84:212–221. doi: 10.1016/j.jtice.2018.01.019. [DOI] [Google Scholar]
- 30.Allard S., Nottle C.E., Chan A., Joll C., von Gunten U. Ozonation of Iodide-Containing Waters: Selective Oxidation of Iodide to Iodate with Simultaneous Minimization of Bromate and I-THM’s. Water Res. 2013;47:1953–1960. doi: 10.1016/j.watres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Xue Y., Tang Y., Li X., Chen W., Wu Y., Che G., Li L. Bromate Inhibition during Ozonation of Bromide-Containing Water by the Presence of Mn Incorporated MCM-41. J. Mater. Sci. Eng. 2018;7:460. doi: 10.4172/2169-0022.1000460. [DOI] [Google Scholar]
- 32.Tan X., Wan Y., Huang Y., He C., Zhang Z., He Z., Hu L., Zeng J., Shu D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017;321:162–172. doi: 10.1016/j.jhazmat.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Chen W., Li X., Tang Y., Zhou J., Wu D., Wu Y., Li L. Mechanism insight of pollutant degradation and bromate inhibition by Fe-Cu-MCM-41 catalyzed ozonation. J. Hazard. Mater. 2018;346:226–233. doi: 10.1016/j.jhazmat.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Pinkernell U., von Gunten U. Bromate minimization during ozonation: Mechanistic considerations. Environ. Sci. Technol. 2001;35:2525–2531. doi: 10.1021/es001502f. [DOI] [PubMed] [Google Scholar]
- 35.Yang J., Li J., Dong W., Ma J., Yang Y., Li J., Yang Z., Zhang X., Gu J., Xie W., et al. Enhancement of bromate formation by pH depression during ozonation of bromide-containing water in the presence of hydroxylamine. Water Res. 2017;109:135–143. doi: 10.1016/j.watres.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 36.Fischbacher A., Löppenberg K., von Sonntag C., Schmidt T.C. A New Reaction Pathway for Bromite to Bromate in the Ozonation of Bromide. Environ. Sci. Technol. 2015;49:11714–11720. doi: 10.1021/acs.est.5b02634. [DOI] [PubMed] [Google Scholar]
- 37.Huang X., Deng Y., Liu S., Song Y., Li N., Zhou J. Formation of bromate during ferrate(VI) oxidation of bromide in water. Chemosphere. 2016;155:528–533. doi: 10.1016/j.chemosphere.2016.04.093. [DOI] [PubMed] [Google Scholar]
- 38.Nie Y., Li N., Hu C. Enhanced inhibition of bromate formation in catalytic ozonation of organic pollutants over Fe–Al LDH/Al2O3. Sep. Purif. Technol. 2015;151:256–261. doi: 10.1016/j.seppur.2015.07.057. [DOI] [Google Scholar]
- 39.Xie L., Shang C., Zhou Q. Effect of Fe(III) on the bromate reduction by humic substances in aqueous solution. J. Environ. Sci. 2008;20:257–261. doi: 10.1016/S1001-0742(08)60040-6. [DOI] [PubMed] [Google Scholar]
- 40.Sui M., Sheng L., Lu K., Tian F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B: Environ. 2010;96:94–100. doi: 10.1016/j.apcatb.2010.02.005. [DOI] [Google Scholar]
- 41.Kasprzyk-Hordern B., Ziółek M., Nawrocki J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B: Environ. 2003;46:639–669. doi: 10.1016/S0926-3373(03)00326-6. [DOI] [Google Scholar]
- 42.Nie Y., Hu C., Yang L., Hu J. Inhibition mechanism of BrO3− formation over MnOx/Al2O3 during the catalytic ozonation of 2,4-dichlorophenoxyacetic acid in water. Sep. Purif. Technol. 2013;117:41–45. doi: 10.1016/j.seppur.2013.03.045. [DOI] [Google Scholar]
- 43.Radhakrishnan R., Oyama S.T., Chen J.G., Asakura K. Electron transfer effects in ozone decomposition on supported manganese oxide. J. Phys. Chem. B. 2001;105:4245–4253. doi: 10.1021/jp003246z. [DOI] [Google Scholar]
- 44.Li W., Gibbs G.V., Oyama S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 1. In situ Raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998;120:9041–9046. doi: 10.1021/ja981441+. [DOI] [Google Scholar]
- 45.Li W., Oyama S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. 2. Steady-state and transient kinetic studies. J. Am. Chem. Soc. 1998;120:9047–9052. doi: 10.1021/ja9814422. [DOI] [Google Scholar]
- 46.Xing S.T., Hu C., Qu J.H., He H., Yang M. Characterization and reactivity of MnOx supported on mesoporous zirconia for herbicide 2,4-D mineralization with ozone. Environ. Sci. Technol. 2008;42:3363–3368. doi: 10.1021/es0718671. [DOI] [PubMed] [Google Scholar]
- 47.Yang H., Yang S., Wu L., Liu W. CeXZr1-XO2 mixed oxides applied to minimize the bromate formation in the catalytic ozonation of a filtered water. Catal. Commun. 2011;15:99–102. doi: 10.1016/j.catcom.2011.08.032. [DOI] [Google Scholar]
- 48.Chao P. Ph.D. Thesis. Arizona State University; Tempe, AZ, USA: Dec, 2002. Role of Hydroxyl Radicals and Hypobromous Acid Reactions on Bromate Formation during Ozonation. [Google Scholar]
- 49.Parrino F., Camera-Roda G., Loddo V., Palmisano G., Augugliaro V. Combination of ozonation and photocatalysis for purification of aqueous effluents containing formic acid as probe pollutant and bromide ion. Water Res. 2014;50:189–199. doi: 10.1016/j.watres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Nawrocki J., Kasprzyk-Hordern B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B: Environ. 2010;99:27–42. doi: 10.1016/j.apcatb.2010.06.033. [DOI] [Google Scholar]
- 51.von Gunten U., Pinkernell U. Ozonation of bromide containing drinking waters: A delicate balance between disinfection and bromate formation. Water Sci. Technol. 2000;41:53–59. doi: 10.2166/wst.2000.0115. [DOI] [Google Scholar]
- 52.Nie Y., Hu C., Li N., Yang L., Qu J. Inhibition of bromate formation by surface reduction in catalytic ozonation of organic pollutants over β-FeOOH/Al2O3. Appl. Catal. B: Environ. 2014;147:287–292. doi: 10.1016/j.apcatb.2013.09.005. [DOI] [Google Scholar]
- 53.Chitrakar R., Makita Y., Sonoda A., Hirotsu T. Fe–Al layered double hydroxides in bromate reduction: Synthesis and reactivity. J. Colloid Interface Sci. 2011;354:798–803. doi: 10.1016/j.jcis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Xia Y., Li Q., Qi F., Xu B., Chen Z. Synchronously degradation benzotriazole and elimination bromate by perovskite oxides catalytic ozonation: Performance and reaction mechanism. Sep. Purif. Technol. 2018;197:261–270. doi: 10.1016/j.seppur.2018.01.019. [DOI] [Google Scholar]
- 55.Zhang T., Hou P., Qiang Z., Lu X., Wang Q. Reducing bromate formation with H+-form high silica zeolites during ozonation of bromide-containing water: Effectiveness and mechanisms. Chemosphere. 2011;82:608–612. doi: 10.1016/j.chemosphere.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 56.Gounden A.N., Singh S., Jonnalagadda S.B. Non-catalytic and catalytic ozonation of simple halohydrins in water. J. Environ. Chem. Eng. 2019;7:102783. doi: 10.1016/j.jece.2018.11.028. [DOI] [Google Scholar]
- 57.Song R., Donohoe C., Minear R., Westerhoff P., Ozekin K., Amy G. Empirical modelling of bromate formation during ozonation of bromide containing waters. Water Res. 1996;30:1161–1168. doi: 10.1016/0043-1354(95)00302-9. [DOI] [Google Scholar]
- 58.Siddiqui M., Amy G. Factors Affecting DBP Formation during Ozone-Bromide Reactions. J. AWWA. 1993;85:63–72. doi: 10.1002/j.1551-8833.1993.tb05922.x. [DOI] [Google Scholar]
- 59.Haag W.R., Hoigne J. Ozonation of bromide-containing waters: Kinetics of formation of hypobromous acid and bromate. Environ. Sci. Technol. 1983;17:261–267. doi: 10.1021/es00111a004. [DOI] [Google Scholar]
- 60.Qi S., Mao Y., Lv M., Sun L., Wang X., Yang H., Xie Y.F. Pathway fraction of bromate formation during O3 and O3/H2O2 processes in drinking water treatment. Chemosphere. 2016;144:2436–2442. doi: 10.1016/j.chemosphere.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Kishimoto N., Nakamura E. Bromate Formation Characteristics of UV Irradiation, Hydrogen Peroxide Addition, Ozonation, and Their Combination Processes. Int. J. Photoenergy. 2012;2012:10. doi: 10.1155/2012/107293. [DOI] [Google Scholar]