Table 1.
Structure, trade name, and producer of commercial polyimides (or precursors) tested for their biocompatibility.
| Structure of Polyimide (or Precursor 1) | Trade Name 2; Producer Biocompatibility Studies [References] |
|---|---|
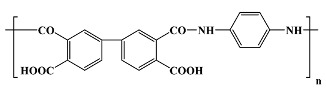
|
PI2611 1; Du Pont in vitro; in vivo [7,16,27,29,30,31,32,33,34,35,36,37,38,39,40] |
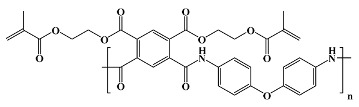
|
Durimide 7000 1; Fujifilm in vitro; in vivo [27,29,36,41,42,43] |
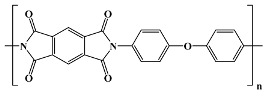
|
Kapton; Du Pont theoretical; in vitro; in vivo [15,20,27,30,44,45,46] |
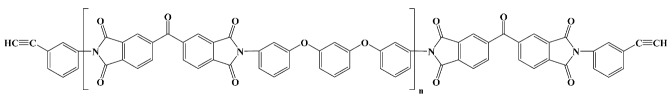
|
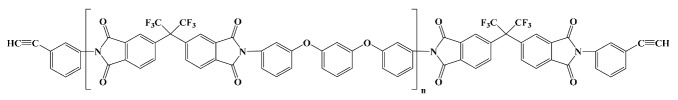
Thermid EL 5010; National Starch and Chemical Co in vitro [15] |
|
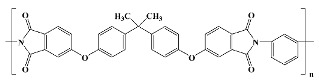
Thermid EL 5512; National Starch and Chemical Co in vitro [15] |
|
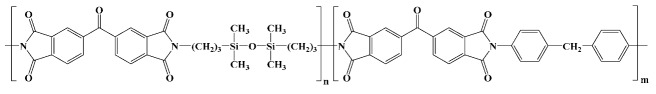
Ultem 1010; GE Plastics in vitro [15] |
|
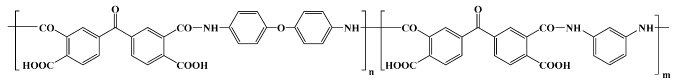
Silbem; GE Plastics in vitro [15] |
|
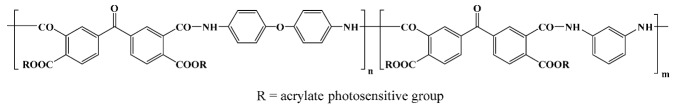
PI2525 1; Du Pont in vitro; in vivo [28,47,48] |
|
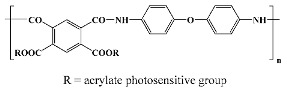
Pyralin PI 2700 1; Du Pont in vitro [28] |
|
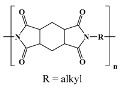
Probimide 7500 1; Fujifilm in vitro; in vivo [49] |
|
Neopulim; Mitsubishi in vitro [19] |
1 structure is represented as the commercial polyimide precursor, but all biocompatibility tests were performed on the imidized form of the polymeric material; 2 common tradenames or abbreviations for the monomers: PMDA (pyromellitic dianhydride), BPDA (biphenyl-tetracarboxylic acid dianhydride), PPD (p-Phenylenediamine), 6FDA (4,4′-(hexafluoroisopropylidene)diphthalic anhydride), ODPA (4,4′-oxydiphthalic anhydride), ODA (4,4′-oxydianiline).
