Table 1.
Chemical structures and bioactivities of eight hit compounds identified by screening.
| Compd. | CAS registry number | Structure | Chemical formula | Molecular weight | IC50 Reporter gene assay (µmol/L) | IC50 Alpha Screen assay (µmol/L) | Percentage inhibition (%) in primary screening (reporter gene assay) | Percentage inhibition (%) in secondary screening (Alpha Screen) | Percentage non-specific inhibition (%) in TruHits screening to eliminate false positives | Medicinal chemist׳s annotation |
|---|---|---|---|---|---|---|---|---|---|---|
| RUS0207-A006 | 497917-11-0 | 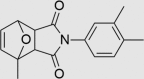 |
C17H17NO3 | 283.322 | 73 | 41 | 76 | 71 | 13 | Related to compound BMS-641988, a novel androgen receptor antagonist for the treatment of prostate cancer Possible co-polymer |
| RUS0202-G005 | 602283-51-2 | 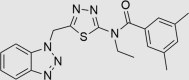 |
C20H20N6OS | 392.477 | 59 | 27 | 99 | 65 | −24 | Class known to have antifungal/antimicrobial activity |
| JK0395-B007 | 852437-97-9 | 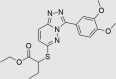 |
C19H22N4O4S | 402.467 | 30 | 25 | 80 | 59 | 13 | Triazolopyridazines patented as protein kinase inhibitors, both broadly, and specifically for inhibition of LRRK2. Inhibition of GABA-A also published |
| RUS0016-B003 | 460073-26-1 | 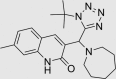 |
C22H30N6O | 394.513 | – | – | 103 | 90 | −9 | Class known to undergo colloidal aggregation (dominant mechanism for artifactual inhibition of proteins*), patented for altering eukaryote lifespan |
| RUS0020-D011 | 460070-80-8 | 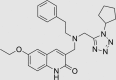 |
C27H32N6O2 | 472.582 | – | – | 103 | 58 | 16 | Class known to undergo colloidal aggregation (dominant mechanism for artifactual inhibition of proteins*), patented for altering eukaryote lifespan |
| RUS0028-G002 | 516450-12-7 | 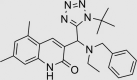 |
C26H32N6O | 444.572 | – | – | 104 | 81 | 13 | Predicted to aggregate by Aggregator Advisor Database*, patented as inhibitors of lymphoid tyrosine phosphatise and urea channel protein |
| RUS0116-H006 | 1164455-04-2 | 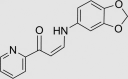 |
C15H12N2O3 | 268.267 | – | – | 96 | 50 | 6 | Patented for altering eukaryote lifespan, related to compounds as antitumor agents against human cancer cell lines. May not be stable in vivo, as it contains an easily hydrolyzed bond |
| RUS0116-G009 | 23576-89-8 | 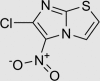 |
C5H2ClN3O2S | 203.606 | – | – | 101 | 148 | 10 | Nitro group too chemically reactive, positive in Ames mutagenicity assay |
The three putative YB-1 inhibitors (RUS0207-A006, RUS0202-G005 and JK0395-B007) are shown in the top three rows with estimated IC50 values. *www.advisor.bkslab.org. –Not applicable.
