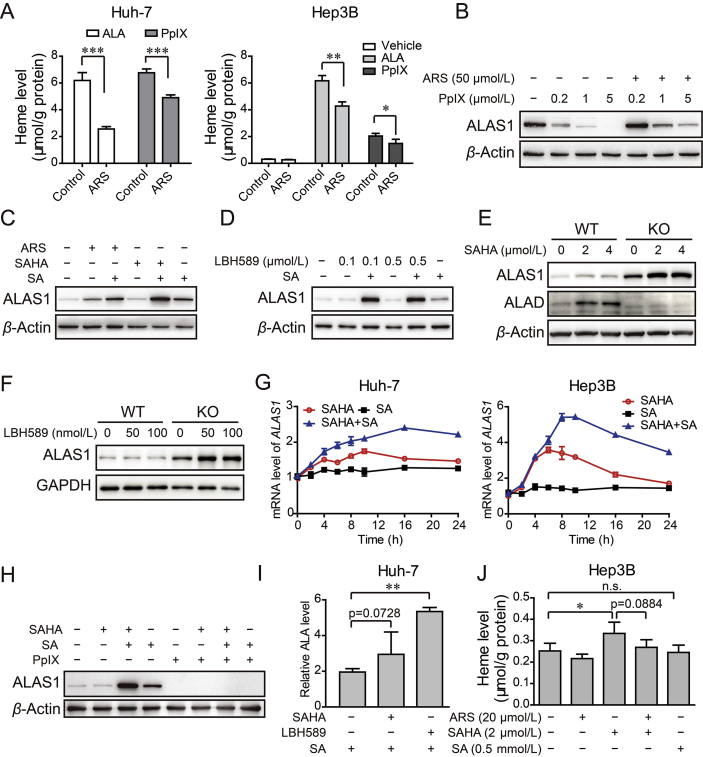
Figure 6.
ARS consumed cellular heme and facilitated HDACi to stably induce synergistic increase in ALAS1 expression. (A) Huh-7 cell (left) and Hep3B cell (right) were pretreated with or without ARS (20 μmol/L) for 12 h, followed with 100 μmol/L ALA in serum free medium or 1 μmol/L PpIX treatment for another 4 h. Heme was measured with the cell lysate. n = 3–4, *P < 0.05, **P < 0.01, ***P < 0.01 (Student's t-test). (B) Representative Western blot analysis of ALAS1 in Hep3B cells treated with various concentration of PpIX in the absence or presence of ARS. (C) The protein levels of ALAS1 in Hep3B cell treated with ARS or SAHA in the absence or presence of SA (0.5 mmol/L). (D) Hep3B cells receiving LBH589 as indicated, in the absence or presence of SA were subjected to Western blot validation of ALAS1. (E)–(F) Hep3B WT and ALAD KO cells were treated with concentrations of SAHA (E) or LBH589 (F) as indicated for 24 h and the amounts of ALAS1 and ALAD were analyzed by Western blot assay. (G) Time course of mRNA level of ALAS1 in Huh-7 cells and Hep3B cells treated with SAHA (4 μmol/L), SA (0.5 mmol/L) or both. (H) Western blot validation of ALAS1 in Hep3B cells treated with SAHA (5 μmol/L), in the absence or presence of SA (0.5 mmol/L) or PpIX (1 μmol/L) or both. (I) HDACi promoted ALA biosynthesis in Huh-7 cells. After 24 h treatment with SAHA (5 μmol/L) or LBH-589 (0.5 μmol/L), cells were lysed for ALA measurement. (J) HDACi promoted heme biosynthesis. Hep3B cells were administrated with ARS, SAHA or the combination in the presence or absence of SA.