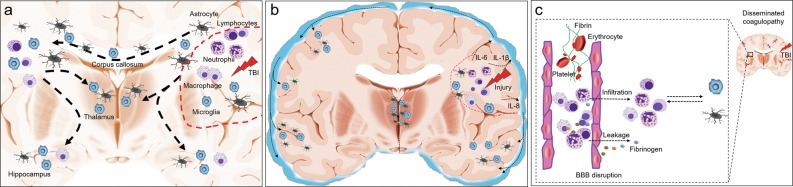
Fig. 2.
Postulated mechanisms that govern the dissemination of brain inflammation after TBI. After the acute primary traumatic brain injury (TBI), immune reactions orchestrated in the injury site are characterized by glial cell activation, the migration of peripheral immune cells, including neutrophils, macrophage, and lymphocytes, as well as the production of many protein products, including cytokines and chemokines. These multiple cellular and protein components induce the formation of focal brain inflammation (a and b: red dashed lines). a TBI commonly induces a remote brain injury, such as diffuse axonal injury, and causes progressive white matter degeneration. Evidence from patients and animal models indicates that globally distributed immune cells, particularly glial cells, are mainly located near or on the white matter, suggesting that brain inflammation is disseminated along the damaged white matter. The disseminated inflammation may further promote white matter degeneration. b Immune mediators are readily released to the cerebrospinal fluid (CSF) through the interstitial fluid, and elevated CSF levels of inflammatory markers, such as IL-6, IL-8, etc., are detected in patients with TBI. Immune cells also enter the CSF, and these cellular and protein components thus disseminate to the central nervous system through the CSF, potentially triggering inflammation in remote areas. c TBI often induces the systemic dysregulation of coagulation characterized by thrombus formation and microbleeding accompanied by BBB disruption. Blood components, including immune cells and proteins such as fibrinogen, are subsequently deposited in the brain parenchyma, orchestrating inflammation. Therefore, inflammation disseminated to the distal areas of the brain after TBI may originate from changes in the blood vessels in the related area