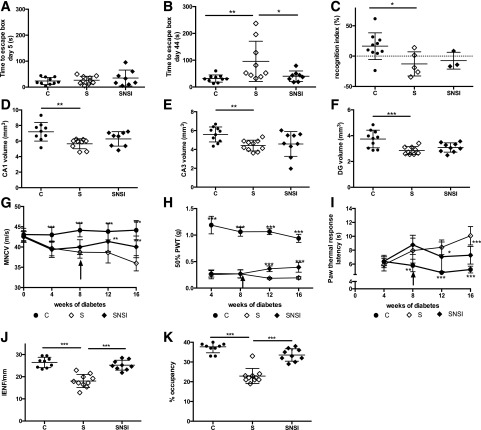
Figure 3.
NSI-189 ameliorates CNS and PNS neuropathy in type 1 diabetic mice. A: Acquisition phase in the Barnes maze (day 5 of training, week 10 of diabetes, week 2 of treatment). B: Memory retention in the Barnes maze (week 16 of diabetes, week 8 of treatment). C: Short-term memory using the object recognition test after 16 weeks of diabetes. CA1 (D), CA3 (E), and DG (F) volume after 16 weeks of diabetes, 8 weeks of treatment. MNCV (G), paw tactile 50% PWT (H), paw thermal response latency (I), paw IENF density (J), and nerve density of the corneal subbasal plexus (K) after 16 weeks of diabetes, 8 weeks of treatment. C, control mice treated daily with vehicle; S, STZ mice treated daily with vehicle; SNSI, STZ mice treated daily with oral NSI-189. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA, followed by the Dunnett post hoc test, for panels A–F, J, and K. *P < 0.05, **P < 0.01, ***P < 0.001 vs. STZ + vehicle mice by repeated-measures ANOVA, followed by the Dunnett post hoc test, for panels G–I (n = 9–10/group).