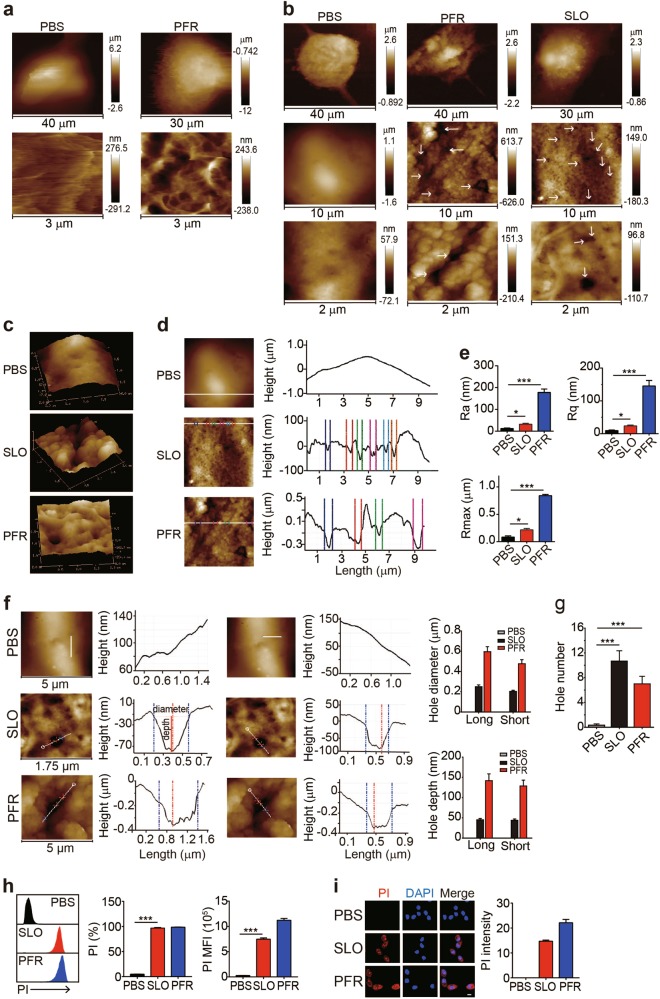
Fig. 1.
Visualization of SLO/perforin-induced pore formation using AFM. a OVA-B16 cells were cultured in 35 mm glass-bottom dishes and treated with PBS or perforin (PFR) (50 U) for 15 min. Cells were then washed with PBS twice and imaged under an atomic force microscope. b–g OVA-B16 cells (1 × 105) were treated with 50 U of SLO or 50 U of PFR isolated from activated human CD8+ T cells for 5 min, fixed with 4% paraformaldehyde and imaged using AFM (b). The 3D topographies of cellular membranes are shown (c). Based on the high-resolution AFM topography data, a regional analysis was performed and surface roughness was analyzed (d). The values of Ra, Rq, and Rmax were calculated (e). The pore diameters along the long and short axes and the pore depth in the membranes of six OVA-B16 cells were calculated within a cellular area of 5 × 5 μm2 (f). For the diameter measurements, the white lines across the pore acted as indicator of the topographies, and the values were calculated between the two dotted blue vertical lines on the curve diagram. The pore depth was defined as the vertical distance between the two red horizontal lines on the curve diagram. Bar, 1 μm. The number of pores was quantified from three 5 × 5 μm2 areas from one cell (n = 6) (g). A pore was defined as a cavity in the plasma membrane with a depth greater than 10 nm. h, i OVA-B16 cells were treated with 50 U of SLO or PFR for 5 min. During the last 30 s of the SLO or PFR treatment, PI (100 μM) was added to the culture medium and PI+ cells were immediately analyzed by flow cytometry (h). Some cells were fixed and observed under a confocal microscope (i). Bar, 20 μm. The experiment was repeated three times. White arrows indicate pores. * p < 0.05 and *** p < 0.001 by one-way ANOVA (e, g, h). The data are presented as the means ± SEM of three independent experiments