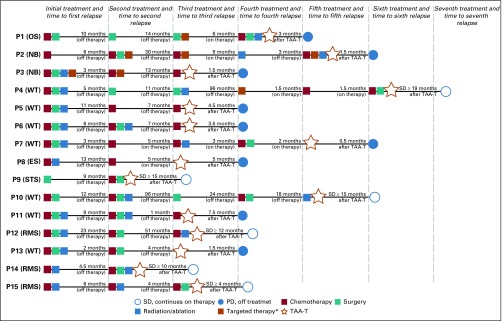
FIG 1.
Treatment summary. Multimodality therapy administered before tumor-associated antigen cytotoxic T cell (TAA-T) infusion. Patients experienced relapsed disease after completion of therapy as well as disease progression while on treatment. (*) Targeted therapy includes the following: denosumab (patient 1 [P1]), dinutuximab (P2 and P3), radiolabeled 131I-MIBG (P2 and P3), lorvotuzumab (P2 and P4). ES, Ewing sarcoma; 131I-MIBG, 131I-meta-iodobenzylguanidine; NB, neuroblastoma; OS, osteosarcoma; SD, stable disease; PD, progressive disease; RMS, rhabdomyosarcoma; STS, soft tissue sarcoma; WT, Wilms tumor.