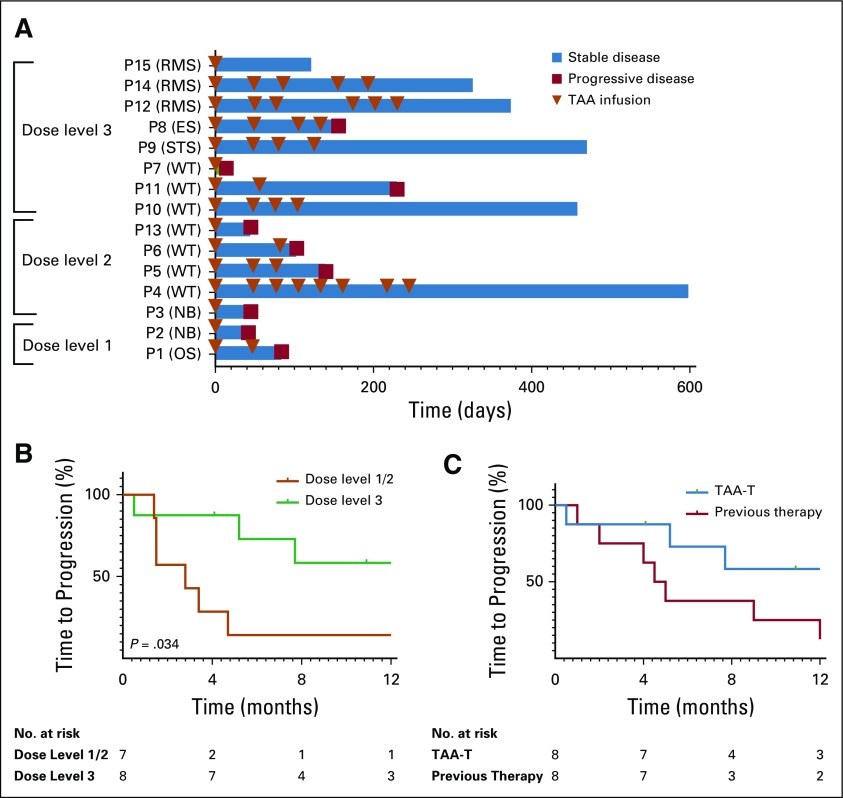
FIG 3.
Disease response. (A) Outcome for evaluable patients who received at least one tumor-associated antigen cytotoxic T cell (TAA-T) infusion. Many patients were able to receive multiple TAA-T infusions without adverse reactions. Eleven of 15 patients met criteria for response, which was defined as stable disease or better at the day 45 evaluation. (B) Median time to progression for patients enrolled in dose level (DL) 1 and 2 (n = 7) was 2.8 months compared with 9.3 months for patients enrolled in DL 3 (n = 8; P = .034). (C) Progression-free survival of patients after TAA-T therapy treated at the highest DL was 73% at 6 months and 58% at 12 months compared with their immediate prior therapy regimen (38% and 25%, respectively; P = .73). ES, Ewing sarcoma; NB, neuroblastoma; OS, osteosarcoma; P, patient; RMS, rhabdomyosarcoma; STS, soft tissue sarcoma; WT, Wilms tumor.