Figure 1.
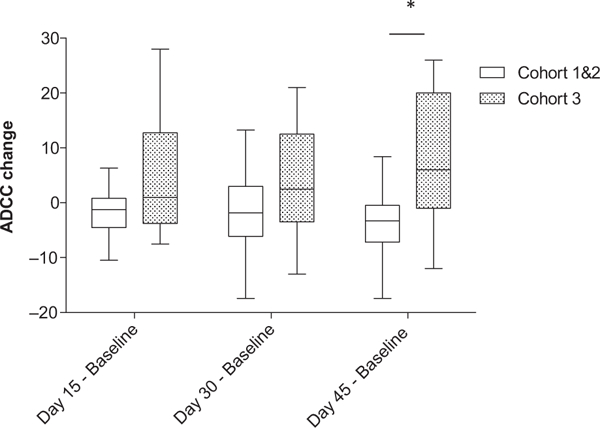
Change in antibody-dependent cellular cytotoxicity between baseline and day 15, day 30, and day 45 for cohort 1 and 2 versus cohort 3. Purified PBMCs from patients enrolled in the trial were incubated overnight with medium supplemented with 10 ng/mL IL12. The lytic activity of patient PBMCs cells was assessed in a standard 4-hour chromium release assay using cetuximab-coated HT29 human colorectal cancer cells. The change in mean percent lysis at 25:1 effector:target ratio for 10 patients enrolled in cohort 1 and 2 and the remaining 12 patients enrolled in cohort 3 between baseline and day 15, baseline and day 30, and baseline and day 45 is shown in box plots, represented as baseline ADCC values subtracted from ADCC values at later time points. The box plots represent the median and interquartile range, with I bars showing the range for each group. *, P = 0.01.
