Figure 3.
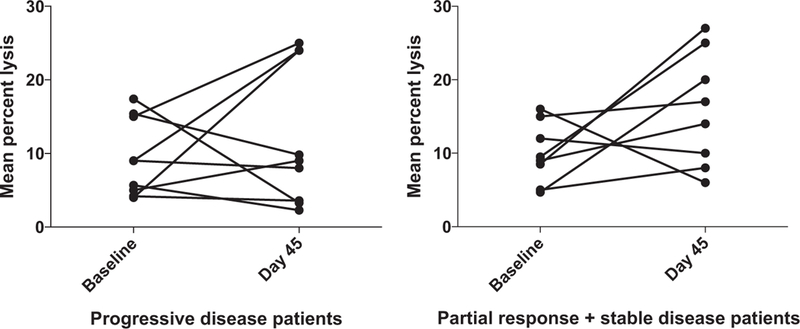
Increased antibody-dependent cellular cytotoxicity is generally correlated to clinical benefit. Purified PBMCs from patients enrolled in the trial were incubated overnight with medium supplemented with 10 ng/mL IL12. The lytic activity of patient PBMCs was assessed in a standard 4-hour chromium release assay using cetuximab-coated HT29 human colorectal cancer cells. The change in mean percent lysis at 25:1 effector:target ratio for 9 patients who had progressive disease (PD) and the 8 patients who experienced clinical benefit (partial response and stable disease, PR + SD) between baseline and day 45 are shown in paired fashion.
