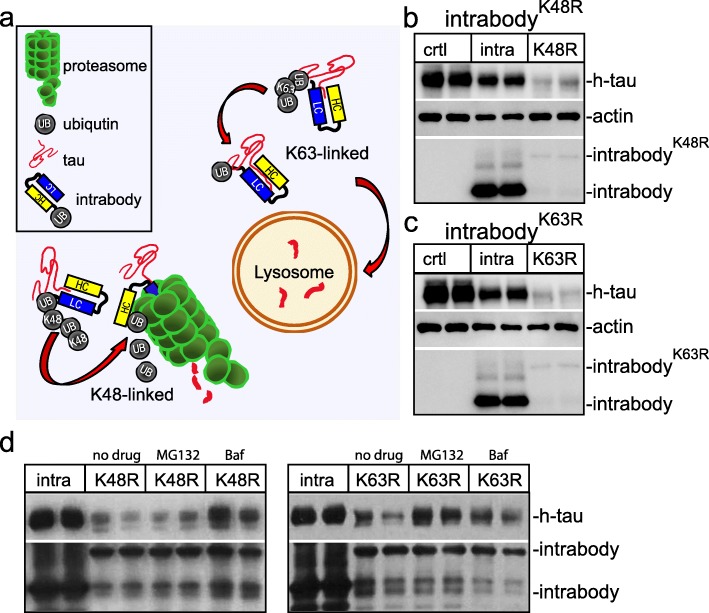
Fig. 1.
Engineering chimeric tau-degrading intrabodies fused to ubiquitin for proteasome or lysosomal-mediated degradation. a Diagram depicts that K48-linked polyubiquitin tau-intrabodies shuttle tau for proteasome degradation, whereas, K63-linked polyubiquitin tau-intrabodies shuttle tau for lysosomal degradation. b Immunoblotting analysis of HEK293t cell lysates co-expressing h-tau together with either a conventional anti-tau intrabody or the anti-tau intrabodyK48R revealed the chimeric anti-tau intrabody fused to ubiquitin harboring a K48R mutation decreased tau protein levels relative to the conventional intrabody or control. Protein levels are relative to actin. The expression of the anti-tau intrabodyK48R also revealed a lower band that potentially corresponds to a cleaved form of the chimeric intrabody. c Immunoblotting analysis of HEK293t cell lysates co-expressing h-tau together with either a conventional anti-tau intrabody or the anti-tau intrabodyK63R revealed the chimeric anti-tau intrabody fused to ubiquitin harboring a K63R mutation decreased tau protein levels relative to the conventional intrabody and control. Similarly, expression of the anti-tau intrabodyK63R also revealed a lower band that potentially corresponds to a cleaved form of the chimeric intrabody. d Immunoblotting analyses of HEK293t cell lysates co-expressing h-tau together with either a conventional anti-tau intrabody, anti-tau intrabodyK63R or anti-tau intrabodyK48R following proteasomal (MG132) or lysosomal (Baf) inhibition