Abstract
Studies of prophylactic cranial irradiation (PCI) focused on elderly patients with small-cell lung cancer (SCLC) are rarely conducted. We aimed to identify whether there is a survival benefit of prophylactic cranial irradiation (PCI) in elderly patients using a single institution’s retrospective data. A total of 234 patients with limited-disease SCLC (LD-SCLC) treated with thoracic chemoradiotherapy were evaluated; of these, 139 patients received PCI. To minimize treatment selection bias, patients were adjusted using the propensity score on factors associated with receipt of PCI. Cox proportional hazard model and Kaplan–Meier analyses were used to identify which subgroup may benefit from PCI. Median follow-up time was 22 months (range 1–150 months). PCI was associated with favorable brain metastasis–free survival, disease-specific survival, and overall survival in the entire population [hazard ratios (HR) 0.588, 95% confidence interval (CI) 0.338–1.024, P = 0.060; HR 0.477, 95% CI 0.331–0.687, P < 0.001; HR 0.543, 95% CI 0.383–0.771, P = 0.001, respectively). However, PCI had no significant relationship with overall survival in patients aged ≥65 years with cT3–4 disease and/or females gender (HR 0.817, 95% CI 0.098–6.849, P = 0.853; HR 1.082, 95% CI 0.114–10.227, P = 0.946, respectively). The benefits and risks of PCI in elderly patients with LD-SCLC need to be scrutinized, especially in those with high T stage tumors and/or females.
Keywords: small-cell lung cancer, prophylactic cranial irradiation, aged, chemoradiotherapy, prognosis
INTRODUCTION
Small-cell lung cancer (SCLC) accounts for 10–15% of all lung cancers, and is characterized by rapid proliferation, early hematogenous dissemination, and high sensitivity to chemotherapy and radiotherapy, compared with other lung cancers [1–5]. Brain metastases are found in ~10% of patients with SCLC at presentation and more than 50% of patients within 2 years of diagnosis, with half of them limited to the brain [5, 6]. Several studies have been conducted on the role of prophylactic cranial irradiation (PCI) in reducing brain metastasis since the 1970s. In some meta-analyses of patients with limited disease SCLC (LD-SCLC) who showed a favorable tumor response after chemoradiotherapy, PCI was associated with improved overall survival and decreased risk of brain metastasis by ~50% [5, 7]. With the exception of some special cases, the addition of PCI is now widely accepted as the standard of treatment when patients with LD-SCLC have reached complete or partial remission after thoracic chemoradiotherapy [8].
However, elderly patients may have lower performance status, which is a poor prognostic factor in SCLC [9]. They have greater risks of death from other comorbid conditions and/or treatment toxicity from treatment for SCLC [10]. In addition, several studies have shown that elderly patients are more at risk for neurocognitive decline following PCI [11]. Considering that the survival benefit from PCI in patients with LD-SCLC is generally seen at 1 year following PCI, the benefit of PCI on overall survival in elderly patients may be less than in younger patients [5].
Studies of PCI focused on elderly patients with SCLC are rarely conducted, and conclusions about the efficacy of PCI in those patients have not been drawn [12, 13]. Elderly patients have also been underrepresented in previous randomized trials of PCI in LD-SCLC, with patients older than 70 and 75 years old representing <10% and <1% of study populations, respectively [5, 7]. We used retrospective data from a single institution to identify whether there is a survival benefit for PCI in elderly patients by identifying which subgroups, if any, benefit from PCI.
MATERIALS AND METHODS
Patients
Records of 320 consecutive patients with biopsy-proven LD-SCLC who were treated with curative intent at —Samsung Medical Center between November 1994 and June 2010 were reviewed. Of these patients, 26 patients visited other clinics for follow-up or missed follow-up, and thus had no available clinical data, 50 patients underwent chemotherapy regimens of etoposide, ifosfamide, and cisplatin (VIP) with concurrent radiotherapy, 10 patients did not complete their chemoradiotherapy course due to poor performance status, or patient refusal of thoracic radiotherapy. These patients were excluded, and the remaining 234 patients with LD-SCLC treated with curative intent using etoposide and cisplatin (EP) and thoracic radiotherapy were evaluated. Of these, 44 patients treated between 1998 and 2001 were enrolled in the Phase II study conducted in our center [14], and 112 patients treated between 2003 and 2010 were enrolled in the randomized open-label Phase III trial [15]. All patients gave written informed consent before treatment. This study was approved by the Institutional Review Board of —Samsung Medical Center (IRB No. SMC-2018-06-052).
Diagnostic examination
All patients underwent pathologic diagnosis of SCLC using bronchoscopic or fine-needle aspiration biopsy. In addition, standard hematologic and biochemical workups, bone marrow aspiration/biopsy if needed, and a radionuclide bone scan were performed if indicated. Brain imaging was performed with magnetic resonance imaging (MRI) at presentation in all patients. Pretreatment imaging of the chest consisted of acontrast-enhanced computed tomography (CT) scan and/or acombined torso 18F-deoxyglucose (FDG) positron emission tomography (PET)/CT scan. The TNM stage system, according to the 8th edition of the American Joint Committee on Cancer, was used for staging.
Treatment
All patients received concurrent chemoradiotherapy doses of at least 44 Gy for primary intrathoracic disease. The gross tumor volume consisted of all known sites of disease, including primary tumor, mediastinal lymph nodes with a short diameter of ≥1 cm, lymph nodes with positive tumor cell sampling, and lymph nodes with increased 18F-FDG standard uptake value on PET/CT. The clinical target volume and planning target volume encompassed the gross tumor volume with adequate margins in all directions. Megavoltage linear accelerators with 4–10 MV photons were used for radiotherapy. Thoracic radiotherapy started either on the day of the first or third chemotherapy cycle (early or late thoracic radiotherapy). Chemotherapy consisted of etoposide (100 mg/m2 on Days 1 to 3) and cisplatin (70 mg/m2 on Day 1), which were administered every 3 weeks for four cycles. Four to eight weeks after completion of thoracic chemoradiotherapy, PCI with a dose of 25 Gy per 10 fractions to the whole brain using opposed lateral beams was suggested to patients with favorable clinical and radiographic responses. However, many patients did not undergo PCI due to a physician’s or patient’s decision in view of several factors, such as neurocognitive toxicity concerns, medical unfitness, and old age.
The work-up and treatment policy for LD-SCLC at our institution have been altered over time. Thoracic radiotherapy schedules were 44 Gy per 22 fractions with once a day fraction between 1994 and 2003, and 52.5 Gy per 25 fractions with once a day fraction between 2003 and 2010. Pretreatment PET/CT scans have been conducted since 2002. Patients were followed every 3 months for 1 year and every 6 months thereafter, with routine chest CT scan and other image examination if clinically indicated.
Statistics
A biostatistician performed the statistical analysis. Baseline patient characteristics were compared using Pearson’s chi-square test for patients who received PCI and those who did not. To evaluate the effect of PCI on patient survival and reduce the impact of treatment-selection bias, significant differences in patient characteristics were adjusted using inverse probability of treatment weighting (IPTW) and propensity score. Univariable and multivariable Cox regression analyses were performed to identify significant predictors of overall survival in the unweighted population. The propensity score model included covariates affecting treatment selection and survival. A logistic regression model involving five covariates was used to estimate the propensity score. The five selected variables were clinical factors that could affect treatment selection. IPTW for PCI was applied to estimate the hazard ratios (HR) regarding treatment effect using the Cox proportional hazard model. Survivals according to receipt of PCI were evaluated using the Kaplan–Meier method and the log-rank test. All statistical analyses were performed using STATA software version 14.0 (Stata Corporation, College Station, TX, USA). A two-sided P-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics and predictors of overall survival
Median follow-up duration was 22 months (range 1–150 months). A total of 204 patients (87%) were male, and the median age was 61 years old (range 34–77 years). The TNM stage was I–II in 54 patients (23%) and III in 180 patients (77%). The tumor volume was measurable in 134 patients, and 69 patients (51%) had a tumor volume of ≥50 ml. PET/CT was performed in 94 patients (40%) as part of the initial staging work-up. The total radiation dose was 44 Gy in 101 patients (43%), and 52.5 Gy in 133 patients (57%). Early thoracic radiotherapy was administered in 149 patients (64%). The complete response (CR) rates were 53.8% and 48.5% in the entire group and patients aged ≥65 years, respectively. In univariable Cox regression analyses, LDH, performance status, cN, clinical stage, radiotherapy dose, the receipt of PCI, and treatment schedule were significantly associated with overall survival (P < 0.001, 0.003, 0.002, 0.002, <0.001, <0.001, and 0.012, respectively) (Supplementary Table 1). Among them, the receipt of PCI was an independent predictor of overall survival (P < 0.001) in multivariable analyses.
Table 1 shows the characteristics of patients who received PCI and those who did not. Of the 234 total patients, 139 patients (59.4%) received PCI and 95 (40.6%) did not. The median age was 60 years (range 34–75 years) and 62 years (range 40–77 years) in the PCI group and the non-PCI group, respectively. Patients were more likely to receive PCI if they were younger (<65 years vs ≥65 years, P = 0.030), had lower lactate dehydrogenase (LDH) level (<400 U/l vs ≥400 U/l, P = 0.006), had favorable performance status (0 vs 1–2, P = 0.054), had higher thoracic radiotherapy dose (52.5 Gy vs 44 Gy, P < 0.001), and received late thoracic radiotherapy (late vs early, P = 0.004).
Table 1.
Patient characteristics
| Characteristics | Total | Patients aged ≥65 years | ||||
|---|---|---|---|---|---|---|
| Non-PCIa (n = 95) | PCIa (n = 139) | P-value | Non-PCIa (n = 35) | PCIa (n = 33) | P-value | |
| Gender | 0.469 | 0.651 | ||||
| Male | 81 (85.3) | 123 (88.5) | 31 (88.57) | 28 (84.85) | ||
| Female | 14 (14.7) | 16 (11.5) | 4 (11.43) | 5 (15.15) | ||
| Age | 0.030 | - | ||||
| <65 years | 60 (63.2) | 106 (76.3) | – | – | ||
| ≥65 years | 35 (36.8) | 33 (23.7) | – | – | ||
| Median (range) | 62 years | 60 years | 67 years | 68 years | ||
| (40–77) | (34–75) | (65–77) | (65–75) | |||
| LDH | 0.006 | 0.041 | ||||
| <400 U/l | 34 (36.6) | 76 (55.1) | 10 (28.6) | 17 (53.1) | ||
| ≥400 U/l | 59 (63.4) | 62 (44.9) | 25 (71.4) | 15 (46.9) | ||
| Median (range) | 459 U/l | 388 U/l | 470 U/l | 391 U/l | ||
| (237–1402) | (224–1078) | (269–758) | (237–927) | |||
| Performance Status | 0.054 | 0.594 | ||||
| 0 | 7 (7.4) | 22 (15.8) | 2 (5.7) | 3 (9.1) | ||
| 1–2 | 88 (92.6) | 117 (84.2) | 33 (94.3) | 30 (90.9) | ||
| cT | 0.565 | 0.242 | ||||
| 0–2 | 80 (84.2) | 113 (81.3) | 32 (91.4) | 27 (81.8) | ||
| 3–4 | 15 (15.8) | 26 (18.7) | 3 (8.6) | 6 (18.2) | ||
| cN | 0.274 | 0.346 | ||||
| 0–1 | 20 (21.1) | 38 (27.3) | 10 (28.6) | 13 (39.4) | ||
| 2–3 | 75 (78.9) | 101 (72.7) | 25 (71.4) | 20 (60.6) | ||
| Stage | 0.543 | 0.492 | ||||
| I–II | 20 (21.1) | 34 (24.5) | 10 (28.6) | 12 (36.4) | ||
| III | 75 (78.9) | 105 (75.5) | 25 (71.4) | 21 (63.6) | ||
| Tumor volume | 0.337 | 0.270 | ||||
| <50 ml | 15 (21.7) | 50 (51.0) | 8 (47.1) | 15 (55.6) | ||
| ≥50 ml | 21 (58.3) | 48 (49.0) | 9 (52.9) | 12 (44.4) | ||
| Median (range) | 53.1 ml | 48.5 ml | 53.2 ml | 45.0 ml | ||
| (21.8–139.8) | (11.4–172.4) | (21.8–115.9) | (11.4–146.4) | |||
| Radiotherapy dose | <0.001 | 0.001 | ||||
| 44 Gy | 62 (65.3) | 39 (28.1) | 21 (60.0) | 7 (21.2) | ||
| 52.5 Gy | 33 (34.7) | 100 (71.9) | 14 (40.0) | 26 (78.8) | ||
| Treatment schedule | 0.004 | 0.475 | ||||
| Early | 71 (74.7) | 78 (56.1) | 20 (57.1) | 16 (48.5) | ||
| Late | 24 (25.3) | 61 (43.9) | 15 (42.9) | 17 (51.5) | ||
PCI = prophylactic cranial irradiation, LDH = lactate dehydrogenase, U/l = units per liter.
aValues are numbers (%).
Survival comparison between the non-PCI group and the PCI group
Of the factors associated with overall survival in univariable or multivariable analyses, marginally different factors between the two treatment groups were chosen as the covariates of the IPTW method. As a background factor, age and gender were added to the covariates. After compensating for the main effect of these factors, including age, gender, LDH level, performance status, thoracic radiotherapy dose, and treatment schedule on selecting PCI using IPTW methods, several prognostic indicators were compared between the non-PCI group and the PCI group (Supplementary Table 2, Table 2). Patients who received PCI had significantly more favorable brain metastasis–free survival, disease-specific survival, and overall survival compared with those who did not [hazard ratio (HR) 0.588, 95% confidence interval (CI) 0.338–1.024, P = 0.060; HR 0.477, 95% CI 0.331–0.687, P < 0.001; HR 0.543, 95% CI 0.383–0.771, P = 0.001, respectively).
Table 2.
PCI effect on brain metastasis–free survival, disease-specific survival, and overall survival
| Before IPTW | After IPTW | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Brain metastasis–free survival | ||||
| Overall | 0.621 (0.371–1.042) | 0.071 | 0.588 (0.338–1.024) | 0.060 |
| <65 years | 0.456 (0.262–0.792) | 0.005 | 0.504 (0.277–0.918) | 0.025 |
| ≥65 years | 0.469 (0.094–2.347) | 0.357 | 0.638 (0.135–3.012) | 0.570 |
| Disease-specific survival | ||||
| Overall | 0.451 (0.321–0.634) | <0.001 | 0.477 (0.331–0.687) | <0.001 |
| <65 years | 0.392 (0.263–0.585) | <0.001 | 0.445 (0.289–0.684) | <0.001 |
| ≥65 years | 0.598 (0.308–1.161) | 0.129 | 0.580 (0.286–1.178) | 0.132 |
| Overall survival | ||||
| Overall | 0.439 (0.322–0.600) | <0.001 | 0.543 (0.383–0.771) | 0.001 |
| <65 years | 0.406 (0.280–0.590) | <0.001 | 0.545 (0.358–0.828) | 0.005 |
| ≥65 years | 0.534 (0.298–0.958) | 0.035 | 0.544 (0.291–1.020) | 0.058 |
PCI = prophylactic cranial irradiation, IPTW = inverse probability treatment weight, HR = hazard ratio, CI = confidence interval.
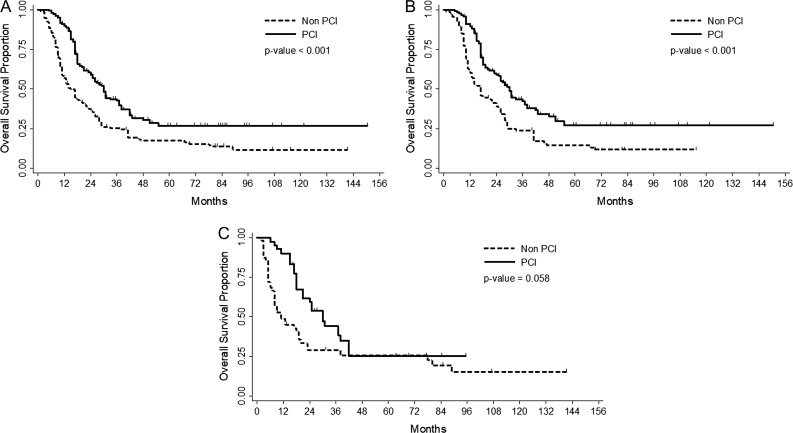
In the age-subgroup analyses, PCI was also significantly associated with brain metastasis–free survival, disease-specific survival, and overall survival in patients aged <65 years (P = 0.025, <0.001, and 0.005, respectively). However, in patients aged ≥65 years, PCI was not significantly associated with any prognostic indicator (P = 0.570, 0.132 and 0.058, respectively). In the entire group, 2-year overall survival rates were 59.13% with PCI and 35.94% without PCI (P < 0.001) (Fig. 1A). The 2-year overall survival rates were also better with PCI (59.07% with vs 38.83% without PCI, P < 0.001) in patients aged <65 years (Fig. 1B). The use of PCI was associated with a borderline trend toward improved overall survival in patients aged ≥65 years (59.32% with vs 29.15% without PCI, P = 0.058) (Fig. 1C).
Fig. 1.
Overall survival by implementation of PCI according to age group: All (Fig. 1A), patients aged <65 years (Fig. 1B) or ≥ 65 years (Fig. 1C).
In both CR and non-CR groups, PCI was associated with favorable overall survival (P = 0.022 and 0.002, respectively) (Supplementary Fig. 1). Similar trends were observed in patients aged <65 years (P = 0.002 and 0.081 for CR and non-CR groups, respectively), but not in patients aged ≥65 years (P = 0.990 and 0.022 for CR and non-CR groups, respectively).
Risk factor for brain metastasis
In univariate Cox-regression analysis, risk factors for brain metastasis were young age and high N stage in unweighted data (P = 0.014 and 0.041, respectively) (Table 3). After IPTW methods, gender and age had significant relationships with brain metastasis (P = 0.027 and 0.034, respectively). cT stage and implementation of PCI was marginally associated with brain metastasis (P = 0.091 and 0.060, respectively). In multivariate Cox-regression analysis, only age was statistically significant (P = 0.167, 0.037, 0.253 and 0.068 for gender, age, cT stage and implementation of PCI, respectively).
Table 3.
Risk factor analysis for brain metastasis
| Variable | Before IPTW | After IPTW | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender (male) | ||||
| Female | 0.707 (0.367–1.362) | 0.301 | 0.500 (0.270–0.368) | 0.027 |
| Age (<65 years) | ||||
| ≥ 65 years | 0.394 (0.187–0.831) | 0.014 | 0.418 (0.187–0.938) | 0.034 |
| LDH (<400 Ul) | ||||
| ≥400 Ul | 1.120 (0.671–1.870) | 0.664 | 1.240 (0.703–2.187) | 0.458 |
| Performance status (0) | ||||
| 1–2 | 2.458 (0.891–6.783) | 0.083 | 1.788 (0.554–5.773) | 0.331 |
| cT (0–2) | ||||
| 3–4 | 1.407 (0.747–2.652) | 0.291 | 1.787 (0.894–3.573) | 0.101 |
| cN (0–1) | ||||
| 2–3 | 1.987 (1.029–3.836) | 0.041 | 1.452 (0.731–2.884) | 0.286 |
| Stage (I, II) | ||||
| III | 1.810 (0.938–3.495) | 0.077 | 1.305 (0.660–2.580) | 0.444 |
| Tumor volume (<50 ml) | ||||
| ≥50 ml | 0.879 (0.440–1.756) | 0.714 | 0.909 (0.413–2.000) | 0.812 |
| Radiotherapy dose (52.5 Gy) | ||||
| 44 Gy | 1.239 (0.744–2.065) | 0.410 | 0.990 (0.563–1.742) | 0.973 |
| PCI (Undone) | ||||
| Done | 0.621 (0.370–1.042) | 0.071 | 0.588 (0.338–1.024) | 0.060 |
| Treatment schedule (Early) | ||||
| Late | 1.131 (0.669–1.914) | 0.646 | 1.033 (0.547–1.956) | 0.918 |
IPTW = inverse probability treatment weight, HR, = hazard ratio, CI = confidence interval, LDH = lactate dehydrogenase, U/l = units per liter, PCI = prophylactic cranial irradiation.
Subgroup analyses in elderly patients
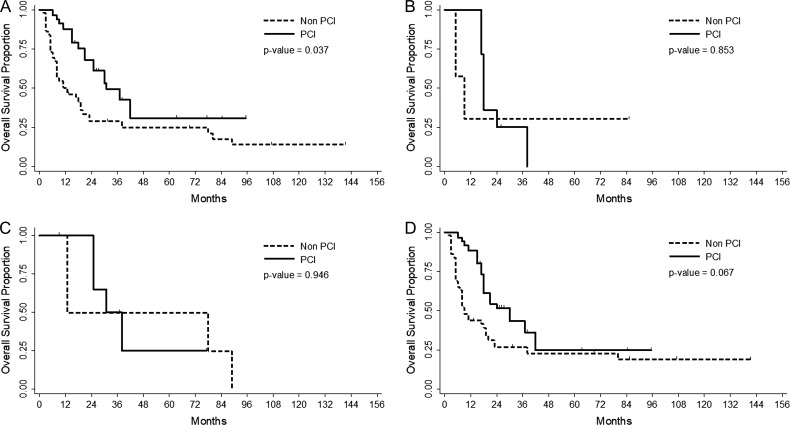
We focused on patients aged ≥65 years and subdivided these patients by gender and cT stage, which showed a relationship with brain metastasis (Table 4, Fig. 2). Implementation of PCI was associated with better overall survival in patients with cT0–2 disease (HR 0.480, 95% CI 0.241–0.955, P = 0.037). Male patients had a tendency for favorable overall survival with PCI (HR 0.539, 95% CI 0.278–1.044, P = 0.067). However, patients with cT3–4 disease and/or females had no significant survival benefit from PCI (P = 0.853 and 0.946, respectively).
Table 4.
PCI effect on overall survivals in patients aged ≥65 years according to cT stage and gender
| Before IPTW | After IPTW | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| cT0–2 | 0.473 (0.248–0.903) | 0.023 | 0.480 (0.241–0.955) | 0.037 |
| cT3–4 | 0.869 (0.158–4.760) | 0.872 | 0.817 (0.098–6.849) | 0.853 |
| Female | 1.814 (0.183–17.857) | 0.610 | 1.082 (0.114–10.227) | 0.946 |
| Male | 0.497 (0.267–0.924) | 0.027 | 0.539 (0.278–1.044) | 0.067 |
IPTW = inverse probability treatment weight, HR = hazard ratio, CI = confidence interval.
Fig. 2.
Overall survival by implementation of PCI for patients aged ≥ 65 years with cT0–2 (Fig. 2A), cT3–4 disease (Fig. 2B), female (Fig. 2C), and male (Fig. 2D).
Patterns of failure and cause of death
In-field failure occurred in 83 patients (35.4%) as the first site of failure (failure-free survival at 2 years: 59.7%). Isolated nodal failure was developed in only 11 patients (4.7%, failure-free survival at 2 years: 95.4%). Non-regional intrathoracic failure was developed in 58 patients (24.8%, failure-free survival at 2 years: 71.4%). Extra-thoracic failure was the most common pattern of failure, and was developed in 109 patients (46.6%, failure-free survival at 2 years: 53.5%). The sites of extra-thoracic failure were brain in 69 patients (29.4%), bone in 46 (19.6%), liver in 41 (17.5%), adrenal gland in 32 (13.6%), retroperitoneum in 16 (6.8%), and soft tissue in 7 (2.9%).
A total of 168 patients died: due to the disease progression in 144 patients, new primary non-small-cell lung cancer in 2, cardiovascular disease in 3, idiopathic pulmonary disease in 2, neutropenic fever in 2, and unknown cause in 5, respectively.
DISCUSSION
In this study, PCI was associated with favorable brain metastasis–free survival, disease-specific survival, and overall survival after IPTW methods in the whole group. The use of PCI was associated with a borderline trend toward improved overall survival in patients aged ≥65 years. However, there was no significant correlation between receipt of PCI and overall survival in elderly patients with high T stage tumors and/or female gender.
The effectiveness of PCI in patients with LD-SCLC has been demonstrated by several clinical studies. Representatively, Auperin et al. established the value of PCI in a meta-analysis of seven randomized trials of 987 patients who achieved complete remission after chemotherapy between 1977 and 1995 [5]. The incidence of brain metastases was significantly reduced by PCI (relative risk 0.46; 95% CI 0.38–0.57), and the 3-year cumulative incidence of brain metastases was also significantly decreased (33% vs 59%). Furthermore, mortality was decreased with PCI (relative risk 0.84; 95% CI 0.73–0.97), which corresponds to a 5.4% increase in the 3-year survival rate. Similar results were obtained in a second meta-analysis that evaluated 1547 patients from 12 randomized trials [7]. However, previous studies have some limitations in light of recent clinical practice. They included very heterogeneous patient populations, including patients who did not receive thoracic radiotherapy, those treated with a variety of chemotherapeutic regimens as well as EP (current standard regimen for SCLC), and those who received different PCI radiation doses. MRI, which is more sensitive than CT in detecting brain metastases, was rarely used in the studies included in the previous two meta-analyses. Furthermore, previous studies rarely included elderly patients, with patients >70 and >75 years comprising <10% and <1% of patients, respectively. The question remains as to whether the benefits of PCI for survival are maintained in the elderly population.
Some studies have evaluated the survival benefit of PCI in elderly patients with LD-SCLC, but the results were not conclusive. Eaton et al. analyzed the effect of PCI on overall survival in 1926 patients aged ≥70 years diagnosed with LD-SCLC between 1988 and 1997 using the Surveillance, Epidemiology, and End Results (SEER) database [16]. Of these, 138 patients (7.2%) who received PCI had a significantly higher overall survival rate at 2 years and 5 years than those who did not receive PCI (33.3% vs 23.1% and 11.6% vs 8.6%, respectively (P = 0.028)). The survival benefit from receiving PCI was maintained in patients aged ≥75 years (P = 0.013), but not in patients aged ≥80 years (P = 0.543). Rule et al. examined the effect of PCI in 155 patients aged ≥70 years with SCLC (84 patients (54.2%) with LD-SCLC and 71 patients (45.5%) with ES-SCLC, respectively) from a pooled analysis of four prospective trials [12]. Of these, 91 patients (58.7%) who received PCI had better survival than patients who did not receive PCI (median survival 12.0 months vs 7.6 months, P = 0.001). The authors supported the role of PCI in elderly patients with SCLC because it was associated with a significant survival advantage in the entire patient population. However, in subgroup analysis, there was no significant difference in overall survival between PCI and non-PCI patients in the LD-SCLC cohort (median survival 12.2 months vs 16.0 months, P = 0.763). It can be inferred that the survival benefit from receiving PCI in the entire elderly patient group is mainly due to the effect of PCI in patients with ES-SCLC, but not due to the effect of patients with LD-SCLC. Farooqi et al. retrospectively analyzed 658 patients who received chemoradiotherapy for LD-SCLC treated at a single institution, of which 364 patients (55.3%) received PCI [13]. Patients who received PCI had less brain metastasis (HR 0.54, 95% CI 0.39–0.76, P < 0.001) and reduced risk of death (HR 0.73, 95% CI 0.61–0.88, P = 0.001) in the entire group. Among 151 patients (22.9%) aged ≥70 years, receipt of PCI had a borderline trend toward improved 2-year overall survival when tumor size was <5 cm (62.5% vs 35.8%, P = 0.056), but not when tumor size was ≥5 cm (39.4% vs 40.9%, P = 0.739). The authors suggested that caution is needed when recommending PCI for elderly patients with large tumors. PCI was also not associated with overall survival improvement in elderly patients with cT Stage 3–4 tumors in a subgroup analysis of the current study. This may be attributed to the hypothesis that elderly patients with large or advanced tumors tend to die prematurely from extracranial disease progression more than an overall survival improvement from PCI.
The following hypothesis can be considered as an explanation for the relatively low effect of PCI on survival in elderly patients. Regardless of whether they have SCLC, the survival time of elderly patients is shorter than that of younger patients due to their high incidence of poor performance status and comorbidities [9, 13]. Therefore, the survival benefit from PCI in elderly patients may be relatively small. There may be an upper age limit for patients who no longer receive survival benefits from PCI. Also, PCI could have a negative impact on neurocognitive function, leading to poor quality of life, especially for elderly patients. In a pooled secondary analysis of two Radiation Therapy Oncology Group (RTOG) trials, PCI was associated with risk of decline in the Hopkins Verbal Learning Test (HVLT) and self-reported cognitive functioning [17]. Patients aged >60 years had higher rates of HVLT-Delayed Recall decline at 12 months following PCI. Welzel et al. evaluated abnormal fractional anisotropy on diffusion tensor imaging and T2-weighted MR images with respect to abnormalities in signal intensity of white matter as markers of radiation damage [18]. Fractional anisotropy decreased in white matter during radiotherapy and 6 weeks after PCI, significantly. Patients aged ≥65 years had a stronger reduction in fractional anisotropy. Ongoing Phase III randomized trials (NCT01780675, NCT02635009 and NCT02397733) exploring PCI with or without hippocampal avoidance are attempts to reduce neurotoxicity from PCI while retaining its effectiveness [19].
Interestingly, receipt of PCI was associated with a borderline trend toward improved overall survival for elderly male patients, but not for elderly female patients in the current study. In a meta-analysis conducted by Auperin et al. to determine whether PCI prolongs survival, PCI reduced the risk of death for 755 men (relative risk, 0.77), whereas it had no effect on 232 women (relative risk, 1.05) [5]. Roengvoraphoj et al. evaluated 179 patients with LD-SCLC treated with definitive chemoradiotherapy to determine whether female gender is a prognostic factor [20]. Female patients had better median overall survival than male patients (20 months vs 14 months, respectively, P = 0.021), and the authors suggest that a higher rate of metachronous brain metastasis in males may be the cause (40/110 men vs 18/69 women, P = 0.03). Previous studies on female gender as a prognostic factor in patients with LD-SCLC did not provide the results of subgroup analysis between elderly and young patients. Further prospective studies of the effect of PCI and the rate of brain metastasis in elderly female patients may provide more interesting results.
The retrospective nature of our study is subject to a lack of neurocognitive and quality of life outcome data that may be exacerbated by receipt of PCI. Another limitation is that the patient characteristics are not the same between patients who received PCI and those who did not. To compensate for differences in patient characteristics that could affect survival, we tried to minimize differences in characteristics using the IPTW method. Third, the number of elderly patients included in the subgroup analysis was as low as 68 (29%). Due to the small number of elderly patients over 70 years old in the current study, we used age 65 as the cut-off point for defining elderly patients instead of 70 years old, which was used in previous studies. A small number of patients may be considered to have induced non-significant results in this group. However, among elderly patients, PCI was associated with overall survival improvement in patients with low T stage tumors. The lack of significant results in elderly patients may be due to the analysis of patients with high T stage tumors or female. Fourth, the reason for not performing PCI was not evaluated as a limitation of a retrospective study, although it can be related to survival. As a single institution study, the strengths of this investigation include that treatment-related factors such as PCI radiation dose and chemotherapy regimen were constant and all participants underwent a baseline MRI test. Our findings could be applied to other centers, considering that the treatment methods in this study are comparable with the recent clinical practices for LD-SCLC.
Receipt of PCI was not related to overall survival benefits in elderly patients with high T stage tumors, even after showing favorable tumor response to chemoradiotherapy for LD-SCLC in the current study. There was no significant association between receipt of PCI and overall survival in elderly female patients, which requires additional validation study. The benefits and risks of PCI in elderly patients with LD-SCLC need to be scrutinized. Further prospective studies evaluating the efficacy of PCI in elderly patients may provide insight into neurocognitive sequelae and PCI ineffective subgroups specific to elderly patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Mi Hyeon Jin for providing statistical advice for this study.
CONFLICT OF INTEREST
The authors report that there is no conflict of interest relevant to this article.
FUNDING
The authors received no specific funding for this work.
REFERENCES
- 1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaspar LE, Gay EG, Crawford J et al. . Limited-stage small-cell lung cancer (stages I–III): observations from the National Cancer Data Base. Clin Lung Cancer 2005;6:355–60. [DOI] [PubMed] [Google Scholar]
- 3. Park J, Kang MK. Impact of radiation dose on concurrent chemoradiotherapy for limited-stage small-cell lung cancer. Radiat Oncol J 2018;36:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janne PA, Freidlin B, Saxman S et al. . Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002;95:1528–38. [DOI] [PubMed] [Google Scholar]
- 5. Auperin A, Arriagada R, Pignon JP et al. . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84. [DOI] [PubMed] [Google Scholar]
- 6. Le Pechoux C, Dunant A, Senan S et al. . Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): a randomised clinical trial. Lancet Oncol 2009;10:467–74. [DOI] [PubMed] [Google Scholar]
- 7. Meert AP, Paesmans M, Berghmans T et al. . Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2001;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rudin CM, Ismaila N, Hann CL et al. . Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106–11. [DOI] [PubMed] [Google Scholar]
- 9. Bremnes RM, Sundstrom S, Aasebo U et al. . The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung Cancer 2003;39:303–13. [DOI] [PubMed] [Google Scholar]
- 10. Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol 2010;28:4086–93. [DOI] [PubMed] [Google Scholar]
- 11. Nakahara Y, Sasaki J, Fukui T et al. . The role of prophylactic cranial irradiation for patients with small-cell lung cancer. Jpn J Clin Oncol 2018;48:26–30. [DOI] [PubMed] [Google Scholar]
- 12. Rule WG, Foster NR, Meyers JP et al. . Prophylactic cranial irradiation in elderly patients with small cell lung cancer: findings from a North Central Cancer Treatment Group pooled analysis. J Geriatr Oncol 2015;6:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farooqi AS, Holliday EB, Allen PK et al. . Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: do all patients benefit? Radiother Oncol 2017;122:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Ahn YC, Kim HJ et al. . Early concurrent chemoradiotherapy with prolonged oral etoposide and cisplatin for limited-stage small-cell lung cancer. Jpn J Clin Oncol 2003;33:620–5. [DOI] [PubMed] [Google Scholar]
- 15. Sun JM, Ahn YC, Choi EK et al. . Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol 2013;24:2088–92. [DOI] [PubMed] [Google Scholar]
- 16. Eaton BR, Kim S, Marcus DM et al. . Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer 2013;119:3753–60. [DOI] [PubMed] [Google Scholar]
- 17. Gondi V, Paulus R, Bruner DW et al. . Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 2013;86:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welzel T, Niethammer A, Mende U et al. . Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol 2008;29:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez de Dios N, Counago F, Lopez JL et al. . Treatment design and rationale for a randomized trial of prophylactic cranial irradiation with or without hippocampal avoidance for SCLC: PREMER trial on behalf of the Oncologic Group for the Study of Lung Cancer/Spanish Radiation Oncology Group–Radiation Oncology Clinical Research Group. Clin Lung Cancer 2018;19:e693–7. [DOI] [PubMed] [Google Scholar]
- 20. Roengvoraphoj O, Eze C, Niyazi M et al. . Prognostic role of patient gender in limited-disease small-cell lung cancer treated with chemoradiotherapy. Strahlenther Onkol 2017;193:150–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.