FIG 1.
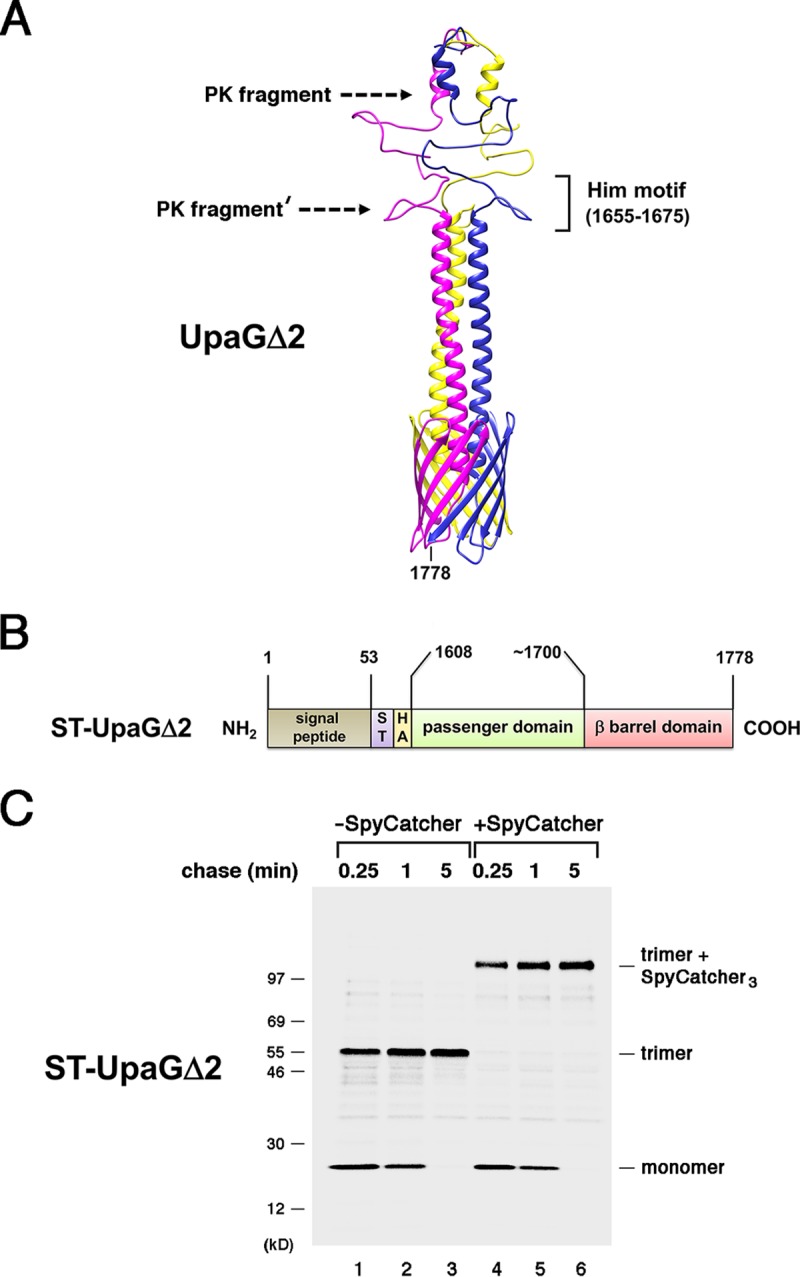
The passenger domains of UpaGΔ2 are secreted rapidly. (A) Homology-based model of the structure of UpaGΔ2 generated using PHYRE2 and GalaxyHOMOMER software. The approximate locations of proteinase K (PK) cleavage sites and a previously identified Him motif (55) are shown. The larger C-terminal fragment was produced by the treatment of native UpaGΔ2 trimer with PK (PK fragment), and the slightly smaller fragment (PK fragment′) was produced by PK treatment of incompletely folded derivatives. The numbers shown here and throughout refer to positions in the full-length UpaG sequence. (B) Illustration of the ST-UpaGΔ2 protein. HA, HA tag; ST, SpyTag. (C) AD202 cells transformed with a plasmid encoding ST-UpaGΔ2 (pRS31) were subjected to pulse-chase labeling. After cells were either incubated with SpyCatcher or mock treated, immunoprecipitations were conducted using an anti-UpaG antiserum and proteins were resolved by SDS-PAGE.
