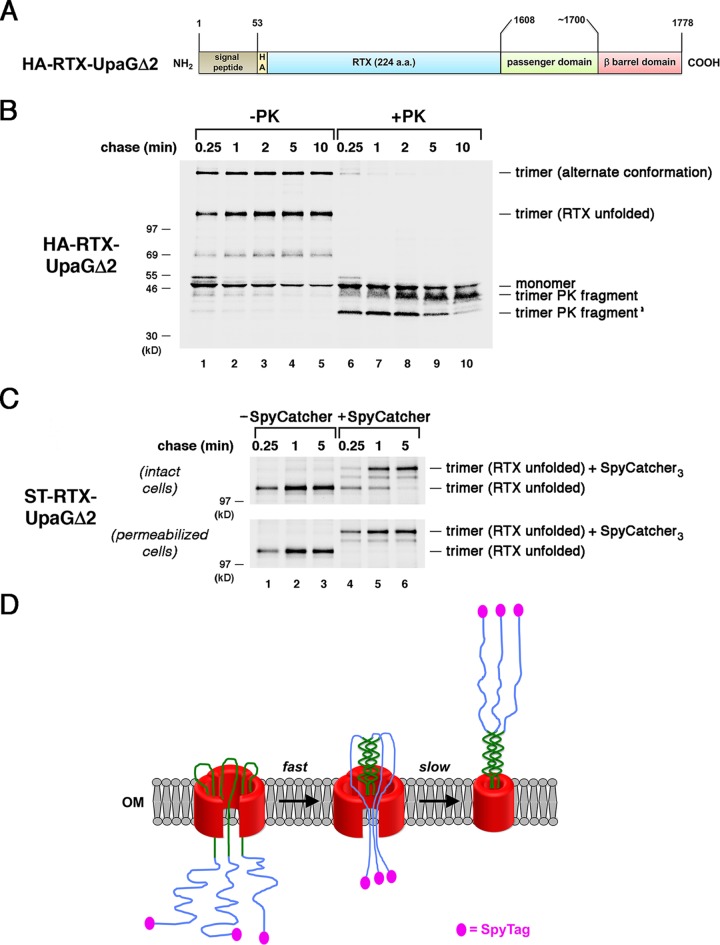
FIG 3.
The presence of an intrinsically disordered segment delays the completion of passenger domain secretion. (A) Illustration of the HA–RTX-UpaGΔ2 protein. HA, HA tag. (B) AD202 cells transformed with a plasmid encoding HA–RTX-UpaGΔ2 (pRS42) were subjected to pulse-chase labeling. After cells were either incubated with PK or mock treated, immunoprecipitations were conducted using an anti-UpaG antiserum and proteins were resolved by SDS-PAGE. (C) AD202 cells were transformed with a plasmid encoding ST–RTX-UpaGΔ2 (pRS43). The experiment represented in panel B was repeated, except that cells were incubated with SpyCatcher instead of PK. The OM of half of the cells was permeabilized prior to the addition of SpyCatcher. (D) Model for the secretion of the ST–RTX-UpaGΔ2 passenger domain. The secretion of the segment of the chimeric passenger domain derived from UpaG is fast and is potentially driven by the formation of a coiled-coil structure. Because the RTX segment cannot fold, its rate of secretion and the level of concomitant surface exposure of the N-terminal SpyTag are considerably lower.