Abstract
Background
Previously, Kuakini Honolulu Heart Program researchers reported that occupational exposure to pesticides was significantly associated with total mortality. The current study examines occupational exposure to pesticides in relation to incident cardiovascular disease, defined as coronary heart disease or cerebrovascular accident.
Methods and Results
With the Occupational Safety Health Administration exposure scale used as an estimate of exposure, statistical analyses were performed on a cohort of 7557 Japanese‐American men from the Kuakini Honolulu Heart Program. Hazard ratios for cardiovascular disease incidence were calculated for various levels of pesticide exposure using Cox proportional hazards models. In the first 10 years of follow‐up, a positive association was observed between age‐adjusted cardiovascular disease incidence and high levels of pesticide exposure (hazard ratio=1.46, 95% CI=1.10‐1.95, P=0.009). This relationship remained significant after adjustment for other cardiovascular disease risk factors (hazard ratio=1.42, 95% CI=1.05‐1.92, P=0.021). No significant association for coronary heart disease or cerebrovascular accident incidence with pesticide exposure was observed when examined separately, possibly due to a smaller number of events.
Conclusions
These findings suggest that occupational exposure to pesticides may play a role in the development of cardiovascular diseases. The results are novel, as the association between occupational exposure to pesticides and cardiovascular disease incidence has not been examined previously in this unique cohort.
Keywords: cardiovascular disease, cerebrovascular accident, coronary heart disease, Honolulu Heart Program, Honolulu‐Asia Aging Study, occupational exposure, pesticide exposure
Subject Categories: Clinical Studies, Basic Science Research, Cardiovascular Disease, Epidemiology, Lifestyle
Clinical Perspective
What Is New?
This is the longest longitudinal study of chronic occupational pesticide exposure and its association with cardiovascular diseases, taking into account epidemiologic risk factors for cardiovascular diseases.
High level of occupational pesticide exposure is associated with 10‐year incidence of cardiovascular diseases.
What Are the Clinical Implications?
Health care providers need to be aware of pesticide exposure occupational health risks, especially in the agricultural population.
Long‐ and short‐term chemical exposures, especially to pesticides, need to be documented in individual medical records.
Farm and agricultural workers need to wear personal protective equipment and have their health monitored for cardiovascular disease outcomes.
Introduction
According to the World Health Organization, cardiovascular diseases (CVD) were the number 1 cause of death worldwide and accounted for 31% of all deaths annually.1 Although there are many contributing causes to heart disease, pesticide exposure is associated with increased mortality and may exert some of its effects via the cardiovascular system.2
Pesticides have been shown to be associated with CVD in other studies, especially in subjects wearing little or no proper personal protective equipment. For example, factory workers involved with phenoxy herbicides and chlorophenol production were found to have an increased incidence of circulatory diseases, including ischemic heart disease and diabetes mellitus.3
Agrochemical products are associated with development of myocardial infarction, congestive heart failure, stroke, arrhythmia, and sudden death.2 A study of chlorophenoxy and phenoxy pesticide manufacturing workers found that hypertension was associated with a positive family history but not occupational exposure.4
According to the Hawaii Department of Agriculture in 1969, common pesticides in Hawaii consisted of several classes of organophosphates, organochlorines, insecticides, fumigants, and herbicides.5 Although pesticides were used as chemical warfare agents during World War I and World War II, they were not used commercially until 1945.6 Most of these chemicals have since been banned, such as chlordane, DDT, dieldrin, heptachlor, hexachlorobenzene, and toxaphene, as they are persistent organic pollutants. Pesticides, depending on their solubility, undergo degradation into different metabolites but may persist for decades.7 The jobs that have been associated with pesticide exposure are either agricultural or industrial. These jobs include pesticide applicators, craftsmen, landscapers, forestry workers, factory workers, pesticide manufacturing workers, aircraft mechanics, jet fuel refinery workers, and agricultural workers.
In the Kuakini Honolulu Heart Program (HHP) longitudinal cohort, there have been multiple studies of occupational exposures and association with various diseases such as cancer8, 9 and Parkinson disease5; however, CVD incidence in association with pesticide exposure has not been evaluated previously. One particularly interesting study was the assessment of occupational exposure to chemicals (pesticides, metals, and solvents) in relation to total mortality from circulatory diseases, respiratory diseases, and cancer by Charles et al.9 The causes of death in this cohort were 28.3% from circulatory disease, 32.4% from cancer, 8% from respiratory disease, 13.1% from a combination of diabetes mellitus, digestive disorders, and other diseases, 3.9% from accidental deaths, and 14.4% from undetermined causes.9 Death from respiratory diseases and all types of cancer were associated with all 3 occupational chemical types, whereas deaths from circulatory diseases were only associated with solvents and pesticides. The mortality analyses were broken down into 5‐year intervals. The 15‐year lag time was found to be the most relevant in relation to circulatory disease mortality from exposure to solvents and pesticides.
Most previous studies examining occupational chemical exposure and cerebrovascular accident (CVA), coronary heart disease (CHD), and CVD have looked at CVD mortality only.9, 10 The purpose of this analysis is to determine if there is an association between occupational exposure to pesticides and the incidence of CVD, CHD, and CVA. Our hypothesis is that occupational exposure to pesticides is a risk factor for incident CVD, CHD, and CVA. However, the mechanisms and biochemical pathways by which pesticides cause the development of these circulatory diseases are not yet fully understood.
Materials and Methods
Because of the sensitive nature of the data collected for this study, Health Insurance Portability and Accountability Act (HIPAA) regulations, and institutional review board approval, requests for limited access of the Kaukini Honolulu Heart Program data set may be possible for purposes of reproducing the results or replicating the procedures, with special requirements to address these confidentiality issues.
Study Design and Population
The Kuakini HHP was originally established in 1965 to study CVD in a cohort of middle‐aged Japanese‐American men living on the island of Oahu, Hawaii. The Kuakini HHP enrolled 8006 participants out of 11 000 possible candidates from a listing of World War II Selective Service records.11 Participants were born between 1900 and 1919 either in Japan or Hawaii. Therefore, at the beginning of this study, the population consisted of either Japanese immigrants or second‐generation Japanese‐American men between the ages of 45 and 68 years.5, 9, 10, 12
Occupational data were collected by self‐report at the baseline exam (1965‐1968) and were available for 7994 individuals. Occupations were categorized according to the US Bureau of the Census definitions. This cohort has undergone multiple examinations and had surveillance for all mortality and some morbidity outcomes. Data on incident CVD were available through December 1999, for up to 34 years of follow‐up. All prevalent cases of CVD (CHD or CVA) at baseline were excluded, leaving an analytic sample of 7557 participants. The Institutional Review Board of Kuakini Medical Center approved this study, and participants or proxy informants gave written informed consent at each examination. These analyses were also approved by the Institutional Review Board of the University of Hawaii at Manoa (CHS# 23491).
Predictor Variables: Occupational Exposure
The Occupational Safety and Health Administration scale was created and coded by industrial hygienists at the National Institute of Occupational Safety and Health for permissible exposure limits in relation to chemical exposures and duration of primary and current jobs. The Occupational Safety and Health Administration scale was used to assess intensity level of exposure for each occupation reported in the Kuakini HHP cohort and is the same scale used in previous studies of this cohort.5, 8, 9, 13 Permissible exposure limits determine the maximum amount of chemical exposure a person can be exposed to over a time‐weighted average. The time‐weighted average is the average amount of exposure over a specific period, usually an 8‐hour workday or a 40‐hour work week.13 Intensity scores for metals, pesticides, and solvents served as independent variables based on industry and agricultural occupational exposure variables and time variables (years worked and an individual's age during that job) and were used to measure total occupational chemical exposure.13 Four categories were defined: no exposure (0), low exposure (1), medium exposure (2), and high exposure (3). According to Kashon and Burchfiel, 3 parameters were taken into account in identifying exposure to pesticides, metals, and solvents; these were (1) if exposure of any kind occurred, (2) an estimate of the magnitude/duration of the exposure, and (3) the particular timing of the exposure.13 Details about how the Occupational Safety and Health Administration scale was created have been previously described.13
Outcome Variables: Incident CVD, CHD, and CVA
Longitudinal follow‐up for CHD and CVA incidence was based on a hospital surveillance system, review of death records, periodic examinations, and an autopsy study. Details of the surveillance methods have been described elsewhere.14 The follow‐up for this report includes incident cases of CHD, CVA, and CVD (defined as either CHD or CVA), after the baseline examination (1965‐1968), through 1999, or a total follow‐up of up to 34 years.
Covariates
The risk factors included age (in years), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, total cholesterol (mg/dL), triglycerides (mg/dL), physical activity, alcohol intake (oz/mo), glucose (mg/dL), body mass index, and education (percentage who graduated from high school). These covariates were measured at examinations during different phases of the study, as reported by Charles et al. Level of education, smoking, and alcohol consumption were self‐reported. One of the main goals of the Kuakini HHP was to study risk factors for the development of CVD. Methods for the baseline examinations have been reported elsewhere.15
Statistical Methods
The mean values and standard deviations of the covariates at baseline were compared according to disease status, that is, those who developed incident CVD and those who did not, using general linear models. Baseline covariates were also compared according to levels of exposure to pesticides (no exposure, low to moderate exposure, and high exposure) using general linear models. Rates of cardiovascular disease incidence were calculated per 1000 person‐years, for the first 10 years of follow‐up separately, and for the overall 34‐year follow‐up period, first without adjustment and then after adjustment for age.
Cox proportional hazard models were used to calculate hazard ratios for CVD incidence in the high‐exposure and low‐ and moderate‐exposure pesticide groups, using the no‐exposure group as reference and adjusting for baseline risk factors. Adjustments for risk factors were evaluated in various models. For example, SBP and body mass index were removed from the model to determine whether there were any effects mediated by these known risk factors. SBP (rather than SBP and DBP) was included as a covariate in the Cox models (to avoid problems with colinearity because SBP and DBP are often associated). All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Table 1 compares baseline CVD risk factors in participants who developed incident CVD over the follow‐up period with those who remained free of CVD. Those who developed incident CVD were significantly older (P=0.0038), and had higher levels of body mass index (P<0.0001), SBP (P<0.0001), DBP (P<0.0001), total cholesterol (P<0.0001), triglycerides (P<0.0001), nonfasting glucose (P<0.0001), and smoking pack‐years (P=0.0269). Those who developed incident CVD had significantly lower alcohol consumption (P=0.0004). There were no significant associations with the physical activity index (P=0.1389) or education (P=0.422).
Table 1.
Baseline Cardiovascular Disease Risk Factors by Disease Status (Incident CVD), Kuakini Honolulu Heart Program
| Baseline Risk Factors | No CVD | Incident CVD | P Value |
|---|---|---|---|
| N=5028 | N=2549 | ||
| Age, y | 54.20±5.52 | 54.59±5.60 | 0.0038 |
| Body mass index, kg/m2 | 23.55±3.11 | 24.36±3.04 | <0.0001 |
| Systolic blood pressure, mm Hg | 131.02±19.73 | 139.10±21.68 | <0.0001 |
| Diastolic blood pressure, mm Hg | 80.74±11.18 | 84.85±11.89 | <0.0001 |
| Total cholesterol, mg/dL | 214.91±36.83 | 223.52±39.48 | <0.0001 |
| Nonfasting triglyceride, mg/dL | 223.79±188.54 | 260.81±228.18 | <0.0001 |
| Nonfasting glucose, mg/dL | 157.20±54.26 | 168.50±64.01 | <0.0001 |
| Physical activity index | 32.96±4.56 | 32.79±4.49 | 0.1389 |
| Smoking, pack‐y | 23.30±24.43 | 24.63±24.40 | 0.0269 |
| Alcohol, oz/mo | 14.77±25.15 | 12.65±23.49 | 0.0004 |
| Education, high school % | 48.87% | 49.84% | 0.422 |
Values are means±SD. CVD indicates cardiovascular disease.
Table 2 compares baseline CVD risk factors in those with no exposure, low to moderate exposure, and high exposure to pesticides. Occupational exposure to pesticides at baseline was significantly associated with older age (P for trend<0.0001), higher physical activity index (P for trend<0.0001), and lower nonfasting triglycerides (P for trend 0.0063). There were significant inverse associations with alcohol intake (P for trend=0.0002) and education (P for trend<0.0001). Associations between pesticide groups and DBP were mixed, without a clear trend. No significant associations were observed for body mass index (P for trend=0.1717), SBP (P for trend=0.8243), total cholesterol (P for trend=0.5799), nonfasting glucose (P for trend=0.6308), or pack‐years smoking (P for trend=0.0962).
Table 2.
Baseline CVD Risk Factors by Levels of Pesticide Exposure, Kuakini Honolulu Heart Program
| Exposure Levels | None | Low‐Moderate | High | P Value for Trend |
|---|---|---|---|---|
| N=7016 | N=110 | N=451 | ||
| Risk factors | ||||
| Age, y | 54.18±5.49 | 55.14±5.46 | 56.61±5.99 | <0.0001 |
| Body mass index, kg/m2 | 23.83±3.12 | 24.10±3.11 | 23.63±2.97 | 0.1717 |
| Systolic blood pressure, mm Hg | 133.67±20.62 | 137.09 22.62 | 133.90±22.36 | 0.8243 |
| Diastolic blood pressure, mm Hg | 82.18±11.54 | 83.27±12.45 | 80.88±12.04 | 0.0202 |
| Total cholesterol, mg/dL | 217.78±38.00 | 215.34±44.60 | 218.81±35.46 | 0.5799 |
| Nonfasting triglyceride, mg/dL | 238.18±204.21 | 219.86±163.01 | 210.69±199.86 | 0.0063 |
| Nonfasting glucose, mg/dL | 160.80±58.08 | 169.50±55.21 | 162.15±56.69 | 0.6308 |
| Physical activity index | 32.80±4.52 | 33.48±4.53 | 34.38±4.60 | <0.0001 |
| Smoking, pack‐y | 23.85±24.51 | 25.33±24.24 | 21.86±23.22 | 0.0962 |
| Alcohol, oz/mo | 14.35±24.82 | 12.44±20.43 | 9.83±21.96 | 0.0002 |
| Education, high school % | 50.53±50.00 | 38.18±48.81 | 31.04±46.32 | <0.0001 |
CVD indicates cardiovascular disease.
Table 3 displays unadjusted and age‐adjusted incidence rates of CVD per 1000 person‐years follow‐up by levels of pesticide exposure (none, low‐moderate, and high), stratified for the first 10 years of follow‐up, and for the total follow‐up period of 34 years. The number of subjects in each group is shown. The highest CVD incidence rates observed during the 10‐ and 34‐year follow‐up periods were in the group with highest exposure to pesticides. It was interesting to note that there appeared to be a lower risk of incident CVD among those with low to moderate levels of exposure, compared to those with no exposure, but this protective effect was not statistically significant.
Table 3.
Incidence Rates of Cardiovascular Disease by Pesticide Groups, Kuakini Honolulu Heart Program
| Exposure Level | Total N | Incident CVD | Unadjusted Rates | Age‐Adjusted Rates |
|---|---|---|---|---|
| Incidence rates of CVD for the first 10‐y follow‐up period | ||||
| No exposure | 7016 | 550 | 8.34 | 8.48 |
| Low‐moderate exposure | 110 | 5 | 4.74 | 4.28 |
| High exposure | 451 | 51 | 12.2 | 10.74 |
| Incidence rates of CVD for the 34‐y follow‐up period | ||||
| No exposure | 7016 | 356 | 14.46 | 14.83 |
| Low‐moderate exposure | 110 | 32 | 12.93 | 12.61 |
| High exposure | 451 | 161 | 16.04 | 15.28 |
Incidence rates per 1000 person‐y. CVD indicates cardiovascular disease.
Table 4 displays the Cox regression models for 10‐year CVD incidence, comparing those with high and low‐moderate levels of exposure to pesticides with those with no exposure (reference group). There was no significant increase in incident CVD in the low‐ to moderate‐exposure group. High levels of pesticide exposure were found to be significantly associated with incident CVD in all models except for the model adjusted only for age.
Table 4.
Hazard Ratios From Cox Proportional Hazards Models and 95% CI for the First 10‐Year Follow‐Up Period, Kuakini Honolulu Heart Program
| Models | Low‐Moderate Pesticide Exposure | P Value | High Pesticide Exposure | P Value |
|---|---|---|---|---|
| Unadjusted | 0.57 (0.24‐1.37) | 0.209 | 1.46 (1.10‐1.95) | 0.009 |
| Adjusted for age | 0.54 (0.22‐1.30) | 0.166 | 1.27 (0.95‐1.69) | 0.108 |
| Adjusted for age+risk factors (except BMI or SBP) | 0.54 (0.23‐1.31) | 0.176 | 1.40 (1.04‐1.89) | 0.027 |
| Adjusted for age+risk factors (except BMI) | 0.49 (0.20‐1.19) | 0.116 | 1.42 (1.05‐1.91) | 0.022 |
| Adjusted for age+risk factors (except SBP) | 0.53 (0.22‐1.29) | 0.163 | 1.40 (1.04‐1.89) | 0.028 |
| Adjusted for age+all risk factors | 0.49 (0.20‐1.19) | 0.116 | 1.42 (1.05‐1.92) | 0.021 |
Risk factors include age, body mass index (BMI), systolic blood pressure (SBP), total cholesterol, nonfasting triglycerides, nonfasting glucose, physical activity index, pack‐years smoking, alcohol intake, percentage with high school education.
In addition, we also explored possible interaction effects between each of the variables in the Cox models, including CVD risk factor levels with pesticide levels. We did not find significant interaction effects (data not shown). Also, there were no significant associations for pesticide exposure with incident CVD over 34 years of follow‐up (data not shown).
Figure 1 is a forest plot of the data from Table 4 to display the results in a more visual way.
Figure 1.
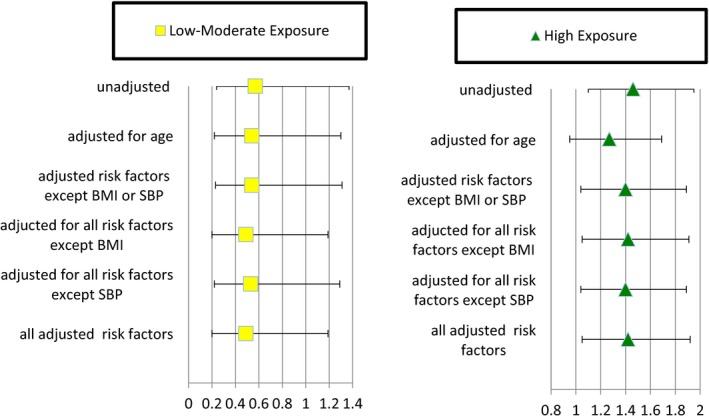
Cardiovascular disease hazard ratios from Cox proportional hazards models for the first 10 years of follow‐up and 95% CIs, Kuakini Honolulu Heart Program. Risk factors include age, body mass index (BMI), systolic blood pressure (SBP), total cholesterol, nonfasting triglycerides, nonfasting glucose, physical activity index, pack‐years smoking, alcohol intake, and percentage with high school education. Yellow squares represent hazard ratios with 95% CIs for low to moderate pesticide exposure. Green triangles represent hazard ratios with 95% CIs for high pesticide exposure.
Figure 2 shows Kaplan‐Meier curves for survival free of incident CVD over the follow‐up period of 34 years by the 3 pesticide exposure groups. In the first 10 years of follow‐up, the group with high exposure to pesticides had the highest incidence of cardiovascular diseases, followed by those with no exposure to pesticides (largest group), and then by the group with low‐moderate exposure. It appears that differentiation between the exposure groups was greatest at 10 years of follow‐up.
Figure 2.
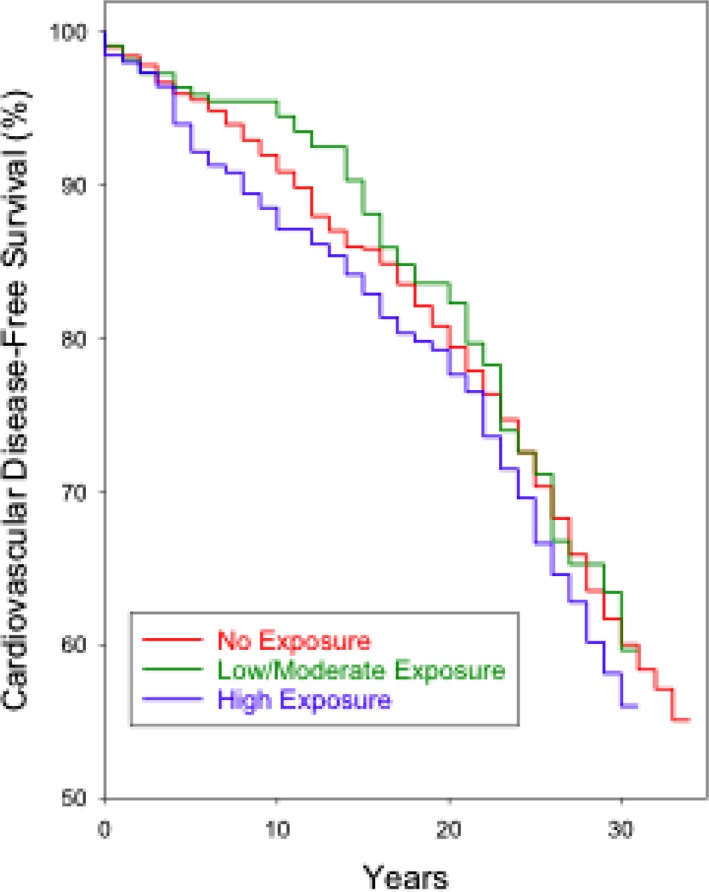
Kaplan‐Meier curves for survival free of incident cardiovascular disease for 3 pesticide‐exposure groups, Kuakini Honolulu Heart Program. X‐axis is exposure time (years). Y‐axis is survival free of incident cardiovascular disease.
It is important to note that we also separately examined the outcomes of incident CHD and CVA and did not find significant associations, probably due to the smaller number of outcomes with inadequate power to detect differences (data not shown).
Discussion
In the current study a high level of pesticide exposure in the first 10 years of follow‐up was found to be significantly associated with CVD incidence after adjustment for all relevant risk factors. In this comprehensive investigation we did not find any association in follow‐up periods >10 years and up to 34 years after exposure. A possible explanation for this observation is that other risk factors associated with aging may mask the effects of toxic pesticide exposure later in life. A study of pesticide factory workers and sprayers of TCDD (tetrachlorodibenzo‐p‐dioxin) reported that the highest susceptibility for developing circulatory diseases occurs 10 to 19 years after exposure to pesticides.3 According to Charles et al, the greatest correlation with chemical exposure (pesticides, metals, and solvents) and total mortality was found 15 years before death in the Kuakini HHP cohort. The Charles et al9 study focused on all‐cause and cause‐specific mortality and did not explore CVD incidence.
The current study found that those in the high‐pesticide‐exposure group had a higher physical activity index than the other groups. This would be consistent with participants in this study, especially those in manual labor jobs, exhibiting a healthy worker effect due to higher levels of physical activity, which may lower the incidence of CVD overall, and this could have attenuated the effect of pesticides on CVD incidence.
We also found that CVD incidence was not associated with low to moderate levels of exposure to pesticides in unadjusted and adjusted models. In fact, our results found nonsignificant trends that suggest low to moderate levels of pesticide exposure may be protective for incident CVD. This could potentially be due to the hormesis principle, which argues that low‐dose exposures to some toxic agents may be protective in some individuals and may stimulate homeostasis of the organism.15 It is thought that low‐dose exposure at nontoxic levels causes stimulation of protective enzymes, which provide enhanced protection against occasional exposure to higher, more toxic levels.16 Examples of hormesis include nutrition, essential vitamins, exercise, sun exposure, calorie restriction, intermittent fasting, pharmaceutical agents, alcohol, and chemicals (basically anything that puts low amounts of stress on the system.17, 18, 19, 20 The benefits of hormesis are limited to the organism's biological plasticity and are thought to be an evolutionary advantage to how the organism's cells adapt to changes in their environment.20
A specific example of the hormesis principle is the effect of moderate alcohol consumption and CVD. The plasma prooxidant activity appears to be due to ethanol metabolism, whereas the antioxidant activity may be due to the absorption of polyphenols in the beverage.21 Many studies have shown a protective effect of moderate alcohol consumption, but alcohol becomes harmful when consumed in larger quantities.18, 20, 21
In 1965, when the study began, proper personal protective equipment for the use of pesticides was not required. Typical routes of pesticide exposure would have been absorption, inhalation, and ingestion. In addition, different occupational exposures may have had a synergistic effect due to exposure to a combination of pesticide chemical classes. Pesticide exposure has previously been linked to development of Alzheimer disease, dementia, cancer, and Parkinson disease in the Kuakini HHP studies.5 A study published in 2015 found an association among hypertension, pesticide exposure, and cognitive decline.22 Previous studies have determined that plantation employees in Hawaii participating in the Kuakini HHP were exposed to organochlorines, organophosphates, and synthetic herbicides.5, 9, 13 Agricultural work would not be the only occupation in which pesticide exposure could occur, as scientists developing agricultural chemicals and technicians in chemical companies would also have occupational chemical exposure.
Based on previous studies, pesticide exposures associated with the development of incident CVD and CHD have been attributed to organophosphates, organochlorines, and herbicides.23, 24, 25, 26, 27 In previous agricultural studies, pesticide exposure was associated with an increase in hypertension, which is also associated with CVD and other diseases.28, 29, 30, 31 Polychlorinated dibenzodioxins and polychlorinated dibenzofurans were found to cause hypertension only in women but not in men.28 PCBs (polychlorinated biphenyls) and chemicals similar to dioxin cause hypertension in men.28 In addition, diazoxonase, used by mosquito sprayers, was found to be associated with increased rates of hypertension.26 The current study adjusted for SBP at baseline to avoid confounding and also conducted analyses without adjusting for SBP to determine if it was a mediating factor and found no effect.
Hung et al32 reported an association between long‐term effects of acute organophosphate poisoning and the development of CVD including arrhythmias, coronary artery disease, and congestive heart failure in middle‐aged men. They suggested that the underlying mechanism leading to CVD in younger people was the disruption in autonomic function due to the organophosphates’ effect on the neurotransmitter that controls cardiac muscles, inducing oxidative stress.32 The acetylcholinesterase enzyme and its neurotransmitter aid in controlling the function of all muscles (skeletal, smooth, and cardiac). Pesticide interference with neurotransmitters is a reasonable explanation because many pesticides are neurotoxic. The Hung et al32 study also reported that the association of organophosphate exposure with CVD was masked by other risk factors as the cohort aged. Individuals heavily exposed to organophosphates had permanent electrocardiographic changes after controls for age and smoking were taken into account.25
The effects of TCDD exposure on serum lipoproteins were still found 20 years after chronic exposure to high dosages in farm workers in the Czech Republic, where exposure was shown to play a role in the development of atherosclerosis and high blood pressure.33 High pesticide exposure affects cells in the liver, leading to oxidative stress and hyperlipidemia and ultimately to cardiovascular morbidity.34 The parent compounds and their metabolites resulting from chronic or acute pesticide poisoning may linger in the body decades after exposure, as some pesticides, such as TCDD, have long half‐lives.
With respect to lipoprotein biomarkers, PCBs and organochlorine exposures have been found to be associated with an increased risk of CVD by reducing arylesterase activity of HDL.35 PCB decreases the PON1 gene activity, which in turn impairs HDL function, thus affecting cholesterol production.35 The PCBs also prevent the oxidization of low‐density lipoproteins.
Wafa et al36 suggested that the inactivation of the PON1 gene causes a decrease in HDL production. Some of the functions of the PON1 gene include reducing oxidative stress, lipid metabolism, and production of HDL. The PON gene product also reduces inflammation and rids the body of pesticides.32, 36, 37, 38, 39 Genetic polymorphisms of the PON1 gene affect production of metabolic enzymes, especially those that aid in the production of cholesterol and neurotransmitters. The PON genes code for enzymes related to both cholesterol production and the breakdown of neurotransmitters. The ability of the PON1 gene to rid the body of pesticides and metals is polymorphism dependent and has been identified in the Turkish population.37, 40 Some PON1 gene alleles are better than others at ridding the body of toxins, and other alleles make subjects more susceptible to developing coronary artery disease. Different populations carry different variants of the PON1, PON2, and PON3 genes. High pesticide exposures lead to cytogenetic effects in the PON genes.39 The PON1 gene is associated with vascular diseases.41 According to Costa et al,41 the PON1 gene aids HDL via high‐affinity reabsorption to leave the liver with assistance from apolipoproteins and phospholipids. A study conducted in the Kuakini HHP cohort found that apolipoproteins predict CHD only for those with low concentrations of HDL.42 Japanese people have polymorphisms that include PON1 584A>G and 172T>A.43 To better understand susceptibility from occupational pesticide exposure, gene‐gene and gene‐environment interactions need to be further investigated. Agirbasli et al37 suggest that diseases dealing with the circulatory system should assess multiple gene‐gene interactions and their cellular pathways and the environment‐gene impact.
The current study has some limitations. One limitation is that the specific pesticides that each participant was exposed to are not known. However, as previously mentioned, documentation by the Hawaii Department of Agriculture from 1969 lists organophosphates, organochlorines, insecticides, and herbicides as being commonly used in agricultural work at that time.5, 44 Another limitation is that the group with a moderate intensity of exposure to pesticides had a small sample size so it was combined with the low‐pesticide‐exposure group, which also contained a small number of subjects. Most participants were in the “no exposure” group. Participants exposed to pesticides were exposed to a wide range of chemical types and classes as well as to other potentially toxic agents. Future studies should look at the combination of both pesticide and solvent occupational exposures in relation to development of CVD. Those 2 chemical types were associated with higher mortality from CVD in a previous study of the same cohort.9 Workers on the job could be potentially exposed to multiple chemicals every day depending on the type of labor and task involved. Another limitation of this study is that, although we were able to adjust for major CVD risk factors, we were unable to adjust for all possible risk factors. The project does not include many of the risk factors identified since the study started in 1965, when many other CVD risk factors were unknown. The data on pesticide exposure were based on self‐report, and this is always subject to memory bias: the exposure information was collected at the beginning of the study before the outcome of CVD occurred, so this would be a nondifferential misclassification bias that would bias toward the null. This study may also have limited generalizability because the study population included only men of Japanese ancestry.
Study strengths include a large sample size and a wealth of information from previous studies published from the same cohort. Another strength of this study is the fact that the cohort was limited to men because some pesticides affect men in a different way than women, and some do not affect women at all.24 Another advantage is that our study population consisted of individuals from the same ethnic group. This is a highly homogeneous group and allows for fewer genetic influences.
Conclusions
Multiple risk factors contribute to the development of cardiovascular diseases, including chemical exposure to pesticides from occupational factors. Although the sample size for the high‐pesticide‐exposure group in our cohort was small compared to the unexposed group, high pesticide exposure was still independently associated with the risk of developing incident CVD. Employees can still have effects related to exposure to chemicals years after their exposure because the pesticides have a long half‐life. By investigating different lag times after exposure, we estimate that the maximum effect of exposure was seen within 10 years.
This study provides valuable insight into chemical occupational exposure and incident cardiovascular diseases and is consistent with the study by Hung et al32 from Taiwan that suggests that acute high dosages of pesticides may contribute to the development of CVD.
Future studies of genetic polymorphisms of the PON1, ‐2, and ‐3 genes and of gene‐ environment interactions need to be further explored in our cohort, as well as others, to determine if there is an association between pesticide exposure and oxidative stress. Some polymorphisms of the PON1 gene have stronger associations with cardiovascular diseases than others; consequently identification of the impacts and mechanisms of specific polymorphisms is needed.
The findings of this research provide insight into the harmful effects of pesticides on the cardiovascular system and confirm a positive association between high levels of pesticide exposure and CVD incidence. These data could be helpful in identifying groups of subjects, such as those involved in agriculture and the manufacturing of pesticides, who may be at higher risk of developing CVD. In addition, they highlight the importance of measures adopted by the National Institute of Occupational Safety and Health, such as protective gear to limit occupational exposure to pesticides, to reduce the increased risk of developing CVD and other diseases associated with pesticide exposure.
Sources of Funding
This study was supported by the National Institutes of Health (National Institute on Aging contract N01AG42149, grant 1R01AG1715501A1, grant U54MD007601, and grant U54MD007584). It was also supported by the National Heart, Lung, and Blood Institute contract N01HC05102, and a National Institute of Neurological Disorders and Stroke grant (1R01NS4126501), and by the National Institute for Occupational Safety and Health (Contract HELD0080060). The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the federal government.
Disclosures
None.
Acknowledgments
The authors thank Dr Cecil Burchfiel for sharing his occupational data analysis expertise and gratefully acknowledge the assistance of the biostatisticians at Kuakini Medical Center with data analysis from the Kuakini Honolulu Heart Program Cohort. The authors thank the J. David Curb Memorial fund, which paid for the publication costs of this manuscript.
(J Am Heart Assoc. 2019;8:e012569 DOI: 10.1161/JAHA.119.012569.)
References
- 1. Fact sheet: cardiovascular fact sheet media centre. World Health Organization: Cardiovascular Fact Sheet; 2015 and updated 2017. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed May 6, 2019. [Google Scholar]
- 2. Sekhotha M. Exposure to agrochemicals and cardiovascular disease: a review. Int J Environ Res Public Health. 2016;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vena J, Boffetta P, Becher H, Benn T, Bueno‐de‐Mesquita HB, Coggon D, Colin D, Flesch‐Janys D, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Pesatori AC, Saracci R, Steenland K, Kogevinas M. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morton WE, Crawford ED, Maricle RA, Douglas DD, Freed VH. Hypertension in Oregon pesticide formulating workers. J Occup Med. 1975;17:182–185. [PubMed] [Google Scholar]
- 5. Petrovitch H, Ross GW, Abbott RD, Sanderson WT, Sharp DS, Tanner CM, Masaki KH, Blanchette PL, Popper JS, Foley D, Launer L, White LR. Plantation work & risk of Parkinson disease in a population‐based longitudinal study. Arch Neurol. 2002;59:1787–1792. [DOI] [PubMed] [Google Scholar]
- 6. Delfino RT, Ribeiro TS, Figueroa‐Villar JD. Organophosphorus compounds as chemical warfare agents: a review. J Braz Chem Soc. 2009;20:407–428. [Google Scholar]
- 7. Somasundaram L, Coats JR. Eds. Pesticide Transformation Products: Fate and Significance in the Environment. 1st ed. Washington, DC: American Chemical Society; 1991; Joel R. Coats, Ch. 2 pesticide degradation mechanisms and environmental activation, volume 459:10–11. [Google Scholar]
- 8. Nelson JS, Burchfiel CM, Fekedulegn D, Andrew ME. Potential risk factors for incident glioblastoma multiforme: the Honolulu Heart Program and Honolulu‐Asia Aging Study. J Neurooncol. 2012;109:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charles LE, Burchfiel CM, Fekedulegn D, Gu JK, Petrovitch H, Sanderson WT, Masaki K, Rodriguez BL, Andrew ME, Ross GW. Occupational exposure to pesticides, metals, and solvents: the impact on mortality rates in the Honolulu Heart Program. Work. 2010;37:205–215. [DOI] [PubMed] [Google Scholar]
- 10. Yano K, McGee D, Reed DM. The impact of elevated blood pressure upon 10‐year mortality among Japanese men in Hawaii: the Honolulu Heart Program. J Chronic Dis. 1983;36:569–579. [DOI] [PubMed] [Google Scholar]
- 11. Worth RM, Kagan A. Ascertainment of men of Japanese ancestry in Hawaii through World War II Selective Service registration. J Chronic Dis. 1970;239:389–397. [DOI] [PubMed] [Google Scholar]
- 12. Kagan A, Popper JS, Rhoads GG. Factors related to stroke incidence in Hawaii Japanese men: the Honolulu Heart Program. Stroke. 1980;11:14–21. [DOI] [PubMed] [Google Scholar]
- 13. Kashon M, Burchfiel C. Occupational exposure in the Honolulu Heart Program: a summary of available data from exam 1 and exam 3. National Institute for Occupational Safety and Health – Health Effects Laboratory Division: Biostatistics Branch; 2002. [Google Scholar]
- 14. Yano K, Reed DM, McGee DL. Ten year incidence of coronary heart disease in the Honolulu Heart Program: relationship to biological and lifestyle characteristics. Am J Epidemiol. 1984;119:653–666. [DOI] [PubMed] [Google Scholar]
- 15. Hively D. Is radiation good for you? Or dioxin? Or arsenic? Discover. 2002;23:74–80. [Google Scholar]
- 16. Mao L, Franke J. Hormesis in aging and neurodegenration—a prodigy awaiting dissection. Int J Mol Sci. 2013;14:13109–13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayes D. Nutritional hormesis and aging. Dose Response. 2009;8:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes D. Nutritional hormesis. Eur J Clin Nutr. 2007;61:147–159. [DOI] [PubMed] [Google Scholar]
- 19. Agathokleos E, Kitao M, Calabrese E. Environmental hormesis and its fundamental biological basis: rewriting history of toxicology. Environ Res. 2018;165:274–278. [DOI] [PubMed] [Google Scholar]
- 20. Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prickett CD, Lister E, Collins M, Trevithick‐Sutton CC, Hirst M, Vinson JA, Noble E, Trevithick JR. Alcohol: friend or foe? Alcoholic beverage hormesis for cataract and atherosclerosis is related to plasma antioxidant activity. Nonlinearity Biol Toxicol Med. 2004;2:353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim SA, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Can inconsistent association between hypertension and cognition in elders be explained by levels of organochlorine pesticides? PLoS One. 2015;10:e0144205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Humblet O, Birnbaum L, Rimm E, Mittleman MA, Hauser R. Dioxins and cardiovascular disease mortality. Environ Health Perspect. 2008;116:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dayton SB, Sandler DP, Blair A, Alavanja M, Beane Freeman LE, Hoppin JA. Pesticide use and myocardial infarction incidence among farm women in the agricultural health study. J Occup Environ Med. 2010;52:693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kristensen TS. Cardiovascular diseases and the work environment: a critical review of the epidemiologic literature on chemical factors. Scand J Work Environ Health. 1989;15:245–264. [DOI] [PubMed] [Google Scholar]
- 26. Samsuddin N, Rampal KG, Ismail NH, Abdullah NZ, Nasree HE. Pesticides exposure and cardiovascular hemodynamic parameters among male workers involved in mosquito control in East Coast of Malaysia. Am J Hypertens. 2016;29:226–233. [DOI] [PubMed] [Google Scholar]
- 27. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure & disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56–65. [DOI] [PubMed] [Google Scholar]
- 28. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23:274–286. [DOI] [PubMed] [Google Scholar]
- 29. Slesinger D. Health status and needs of migrant farm workers in the United States: a literature review. J Rural Health. 1992;8:227–233. [DOI] [PubMed] [Google Scholar]
- 30. Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind Health. 2003;41:149–157. [DOI] [PubMed] [Google Scholar]
- 31. Arrebola JP, Fernández MF, Martin‐Olmedo P, Bonde JP, Martín‐Rodriguez JL, Expósito J, Rubio‐Domínguez A, Olea N. Historical exposure to persistent organic pollutants and risk of incident hypertension. Environ Res. 2015;138:217–223. [DOI] [PubMed] [Google Scholar]
- 32. Hung DZ, Yang HJ, Li YF, Lin CL, Chang SY, Sung FC, Tai SC. The long‐term effects of organophosphates poisoning as a risk factor of CVDs: a nationwide population based cohort study. PLoS One. 2015;10:e0137632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelclová D, Prázny M, Skrha J, Fenclová Z, Kalousová M, Urban P, Navrátil T, Senholdová Z, Smerhovsky Z. 2,3,7,8‐TCDD exposure, endothelial dysfunction and impaired microvascular reactivity. Hum Exp Toxicol. 2007;26:705–713. [DOI] [PubMed] [Google Scholar]
- 34. Imke C, Rodriguez BL, Grove JS, McNamara JR, Waslien C, Katz AR, Willcox B, Yano K, Curb JD. Are remnant‐like particles independent predictors of coronary heart disease incidence? The Honolulu Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:1718–1722. [DOI] [PubMed] [Google Scholar]
- 35. Ljunggren SA, Helmfrid I, Salihovic S, Bavel B, Wingren G, Lindahl M, Karlsson H. Persistent organic pollutants distribution in lipoprotein factors in relation to cardiovascular disease and cancer. Environ Int. 2014;65:93–99. [DOI] [PubMed] [Google Scholar]
- 36. Wafa T, Nadia K, Amel N, Ikbal C, Insaf T, Asma K, Hedi MA, Mohamed H. Oxidative stress, hematological and biochemical alterations in farmers exposed to pesticides. J Environ Sci Health B. 2013;48:1058–1069. [DOI] [PubMed] [Google Scholar]
- 37. Agirbasli M, Güney AI, Ozturhan S, Agirbasli D, Ulucan K, Sevinc D, Kirac D, Ryckman KK, Williams SM. Multifactor dimensionality reduction analysis of MTHRF, PAI‐1, ACE, PON1, and eNOS gene polymorphisms in patients with early onset coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2011;18:803–809. [DOI] [PubMed] [Google Scholar]
- 38. Gugliucci A, Caccavello R, Nassar H, Abu Ahmad W, Sinnreich R, Kark JD. Low protective PON1 lactonase activity in an Arab population with high rates of coronary heart disease and diabetes. Clin Chim Acta. 2015;445:41–47. [DOI] [PubMed] [Google Scholar]
- 39. Da Silva J, Moraes CR, Heuser VD, Andrade VM, Silva FR, Kvitko K, Emmel V, Rohr P, Bordin DL, Andreazza AC, Salvador M, Henriques JA, Erdtmann B. Evaluation of genetic damage in a Brazilian population occupationally exposed to pesticides and its correlation with polymorphisms and metabolizing. Mutagenesis. 2008;23:415–422. [DOI] [PubMed] [Google Scholar]
- 40. Taşkiran P, Cam SF, Sekuri C, Tüzün N, Alioğlu E, Altintaş N, Berdeli A. The relationship between paraoxonase gene‐Leu‐Met (55) and Gln‐Arg (192) polymorphisms and coronary artery disease. Turk Kardiyol Dern Ars. 2009;37:473–478. [PubMed] [Google Scholar]
- 41. Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomics of paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. [DOI] [PubMed] [Google Scholar]
- 42. Sharp DS, Burchfiel CM, Rodriguez BL, Sharrett AR, Sorlie PD, Marcovina SM. Apolipoprotein A‐1 predicts coronary heart disease only at low concentrations of high‐density lipoproteins cholesterol: an epidemiological study of Japanese‐Americans. Int J Clin Lab Res. 2000;30:39–48. [DOI] [PubMed] [Google Scholar]
- 43. Yamada Y, Ando F, Niino N, Miki T, Shimokata H. Association of polymorphism of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community‐dwelling Japanese. J Hum Genet. 2003;48:469–475. [DOI] [PubMed] [Google Scholar]
- 44. Hawaii Department of Agriculture . Evaluation of Pesticide Problems in Hawaii. Honolulu: Hawaii Department of Agriculture; 1969. [Google Scholar]


