Fig. 9.
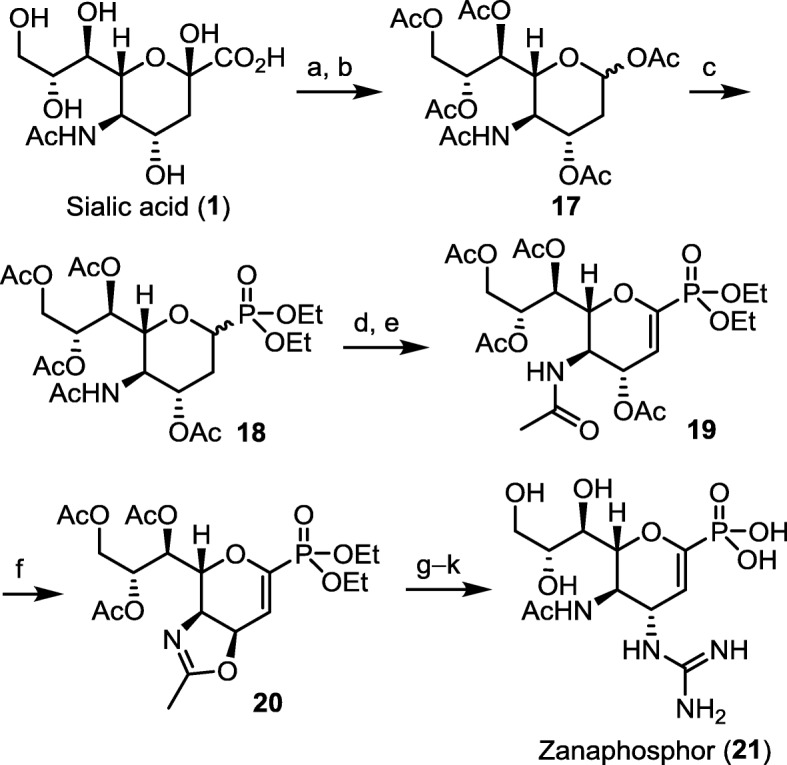
A practical synthesis of zanaphosphor. (a) Ac2O, py, rt., 12 h; (b) 100 °C, 5 h, 50% yield for two steps; (c) TMSOTf, P(OEt)2OTMS, 0 °C to rt., 24 h, 62% yield; (d) NBS, CH2Cl2, hv; (e) py, 50 °C, 1 h, 75% yield for two steps; (f) conc. H2SO4, Ac2O, AcOH, rt., 48 h; 80% yield; (g) TMSN3; (h) H2, Lindlar cat.; (i) MeS-C(=NBoc)NHBoc, HgCl2, Et3N, CH2Cl2; (j) TMSBr, CH2Cl2; (k) MeONa, MeOH, 55% yield for 5 steps. Boc = tert-butoxycarbonyl, NBS = N-bromosuccinimide, py = pyridine, TMS = trimethylsilyl, TMSOTf = trimethylsilyl trifluoromethanesulfonate
