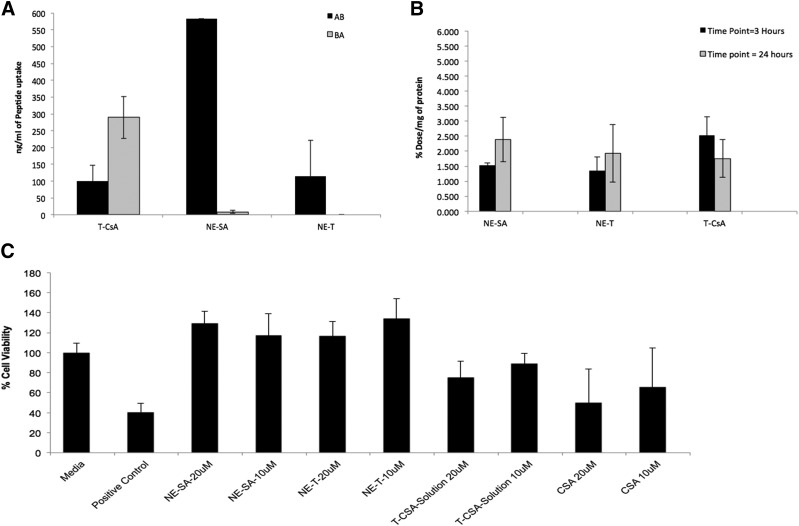
Fig. 1.
(A) T-CSA (cellular transport of cyclosporine in solution), NE-SA (positively charged nanoemulsion), and NE-T (negatively charged nanoemulsion) through RPMI 2650 monolayer cells from AB direction and BA (data presented as mean ± S.D.; n = 3). (B) Intracellular uptake of T-CSA, NE-SA, and NE-T in RPMI 2650 cells at 3 and 24 hours (data are presented as the mean ± S.D.; n = 3). (C) RPMI 2650 cell viability results for CSA nanoemulsion formulations T-CSA, NE-SA, and NE-T at 10 and 20 μM concentrations, respectively, when compared with a solution of CSA at 48 hours. Only media was used as a negative control and treatment with polyethyleneimine was used as a positive control. Data are presented as the mean ± S.D. n = 8.