Abstract
Background
To study the levels of procalcitonin (PCT) in patients with meningitis and control group and compare them with established markers of infection—such as C‐reactive protein (CRP), high‐sensitivity CRP, and WBC—in cerebrospinal fluid (CSF) and assess the possible discriminative role of PCT in the differential diagnosis of meningitis from other noninfectious diseases.
Methods
We studied CSF samples of patients from Intensive Care Unit, Internal Medicine, Neurology, Hematology, and Pediatric departments. The total number of patients included in the study was 58. The samples were divided into three groups: group 1 with bacterial meningitis (BM) central nervous system (n = 19); group 2 with viral meningitis (VM, n = 11); and group 3, control group, with noninfectious diseases (n = 28).
Results
Values of PCT levels >0.5 ng/ml were considered as abnormal. In group 1, mean PCT levels were 4.714 ± 1.59 ng/ml. In group 2, all patients had PCT <0.5 ng/ml (0.1327 ± 0.03 ng/ml). In group 3, the mean PCT levels were <0.1 ng/ml.
Conclusion
PCT values in CSF can be very helpful in distinguishing BM from VM and other noninfectious diseases.
Keywords: procalcitonin, meningitis, bacterial, cerebrospinal fluid
INTRODUCTION
Meningitis is a universal health problem. The laboratory plays a key role in the diagnosis of the disease and subsequently in the appropriate treatment. Nevertheless, there is no test with 100% sensitivity and specificity for early diagnosis of bacterial meningitis (BM).
Procalcitonin (PCT), a protein that consists of 116 amino acids, is considered as a marker of bacterial infections as well as meningitis 1, 2, 3. It is well known that PCT in healthy subjects is produced by the C cells of the thyroid gland and the neuroectodermal cells of lungs, nevertheless in negligible levels (<0.1 ng/ml). Exposure to bacterial LPS (lipopolysaccharide) may lead to a 1,000‐fold increase of PCT in the circulation 4. PCT messenger RNA is ubiquitously and uniformly expressed in many tissues and in response to sepsis is widely upregulated 5. A raised level of PCT in patients with infection has been documented in numerous studies, although cut‐off levels have not been established 6, 7, 8. The above is a difficult task, as the PCT levels vary in different clinical conditions. For example, neonates normally after birth have a peak of PCT that tails off and returns to normal‐low levels, in 48 to 72 hr 9. PCT can also increase in stressful conditions such as surgery, accidents etc. 10. Although the measurement of PCT in serum is now routinely carried out, the significance of PCT in the cerebrospinal fluid (CSF) during inflammation or infection is not fully understood.
A few studies regarding PCT levels in CSF presented conflicting results. Several authors have reported the quantitative evaluation of PCT as a diagnostic marker of BM 2, 11, 12. On the contrary, Shimetani et al. found that CSF's PCT levels in patients with meningitis were not different from patients with noninflammatory central nervous system (CNS) diseases 13. Gendrel et al. reported that CSF's PCT in children with BM and viral meningitis (VM) was undetectable 14. Furthermore, PCT was found to be elevated in the CSF after traumatic brain injury 15. Compared to control subjects, PCT concentration in CSF was significantly increased in patients with Alzheimer's disease 16. In this study, we investigated PCT in CSF and evaluated PCT mean values comparing subjects with BM, VM, and other noninfectious diseases.
MATERIALS AND METHODS
The study group consisted of 58 patients who were recruited from Intensive Care Unit, Internal Medicine, Neurology, Hematology, and Pediatric departments of the General University Hospital of Alexandroupolis. The patients were divided into three groups:
Group 1 with BM (n = 19),
Group 2 with VM (n = 11),
Group 3, control group, with noninfectious diseases (n = 28).
The diagnosis of BM was based on compatible clinical signs and a positive culture of CSF. All CSF samples, in patients with acute meningitis, were tested for the presence of viruses by PCR. In the absence of BM, criteria and identification of viral species by positive PCR patients were enrolled in the second group.
Inclusion criteria for the third group (controls) were absence of clinical manifestations of meningitis and negative CSF for BM and VM. Exclusion criteria included recent CNS surgery, hospital infection, and unavailability for review. Patients who have been transferred to other hospitals were also excluded from the study. The patients from control group were age specific matched with the patients with BM and VM, and were from Internal Medicine, Neurology, and Pediatric departments of the General University Hospital of Alexandroupolis.
The samples of CSF were obtained by lumbal puncture and subsequently were centrifuged, and supernatant was collected and stored at −20°C for measurements of nonroutine parameters in CSF. Before processing, samples were thawed at room temperature for 30 min and by inversion.
The CSF's investigation included leukocyte count, protein, glucose CRP (C‐reactive protein), high‐sensitivity CRP (hs‐CRP) and PCT. Epidemiological studies indicate that enteroviruses are the most common identified cause of acute VM 17, 18, 19. Therefore, genomic amplification by PCR initially was performed for enteroviruses, but subsequently the viral study was extended with PCR for HSV (Herpes simplex virus) 1 and 2, CMV (Cytomegalovirus), and EBV (Epstein–Barr virus).
WBC counts were performed by optical microscopy with a Rosenthal chamber, and PCT was measured by an immunoluminometric assay (Lumi test PCT, Brahms Diagnostic Germany). The threshold for detection of PCT defined by our laboratory is 0.1(ng/ml). Values of PCT levels >0.5 ng/ml were considered as abnormal. CRP and hs‐CRP were measured by nephelometric assay (Beckman Coulter IMAGE 8000). The PCT measurements were performed by scientists with no access to clinical data.
The viral nucleic acid was extracted from 140 μl CSF using the QIAamp Viral RNA kit (Qiagen, Crawley, UK) and eluted in 60 μl, according to the manufacturer's instructions for molecular detection of enteroviruses. Primers used for real‐time PCR amplification of enteroviruses are neENT1 from 430 to 450 bp and neENT2 from 547 to 567 bp, resulting a 123‐bp PCR product. RNA samples were initially held at 95°C for 5 min and consequently at 42°C for 30 min and at 95°C for 5 min. For real‐time PCR, 95°C for 5 min was followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 1 min, and extension at 72°C for 30 sec. Finally, a cycle for dissociation segment was applied, starting with incubation at 95°C for 1 min and then ramping up the temperature from 55 to 95°C. The Brilliant SYBR Green Quantitative PCR Core Reagent kit was used with the Stratagene Mx3005p instrument. Each reaction contained 12.5 μl of 2× master mix, 0.125 μl of sense primer (100 nM), 0.125 μl of antisense primer (100 nM), 0.375 μl of reference dye (30 nM), 6.875 μl nuclease‐free PCR grade water, and 5 μl of specific RT‐reaction product containing the appropriate cDNA. The Brilliant SYBR Green QPCR master mix includes SureStart Taq DNA polymerase, a modified version of Taq2000 DNA polymerase with hot start capability.
The viral DNA was extracted from 200 μl CSF samples using the QIAamp DNA Mini kit according to manufacturer's instructions for molecular detection of HSV 1 and 2 (kit lot 10243010), EBV (kit lot 19546), and CMV (kit lot 70012301). The PCR was performed on LightCycler 2 (Roche Diagnostics, Indianapolis, IN) according to manufacturer's instructions in the Laboratory of Microbiology, Democritus University of Thrace.
Statistical analysis was performed with SPSS version 13 for Windows. To determine the value of the different parameters for the differential diagnosis, the patients were divided into three groups. Student's t test and one‐way ANOVA were used for data that followed Gaussian distribution, while the Mann–Whitney and Kruskal–Wallis tests were used for data that did not follow the Gaussian distribution. For all measurements, a two‐tailed P‐value ≤0.05 was considered as significant. Finally, we calculated sensitivity, specificity, positive (PPV) and negative predictive values (NPV) for PCT test.
RESULTS
The main characteristics of the patients are presented in Table 1. The commonest causative bacteria of BM were as follows: Streptococcus pneumoniae in six subjects and Neisseria meningitidis in five. Other causes included S. aureus, Haemophilus influenze, and Acinetobacter baumannii. The isolated viral species, by real‐time PCR, in all 11 subjects with VM were enteroviruses. All CSF samples of patients with acute meningitis and controls subjects were negative for HSV 1 and 2, EBV, and CMV. The CSF laboratory findings in patients with meningitis and control group are presented in Table 2. Parameters such as leukocyte count and CRP showed significant difference (P < 0.01) among BM, VM, and controls, whereas glucose was significantly decreased (P < 0.001) in BM cases (Table 2).
Table 1.
Clinical Characteristics of Patients on Admission Expressed as Means (±SD) or Numbers (%)
| Variable | BM (n = 19) | VM (n = 11) | Controls (n = 28) |
|---|---|---|---|
| Demographic data | |||
| Age | 41.04 ± 7 | 24.09 ± 12.3 | 30.16 ± 11.54 |
| Male | 12 (63.16) | 8 (72.7) | 20 (71.4) |
| Clinical features | |||
| Headache | 19 (100) | 9 (81.82) | 3 (10.71) |
| Temperature | 18 (94.7) | 11 (100) | 5 (17.86) |
| Vomiting | 6 (31.58) | 5 (45.45) | 2 (7.14) |
| Neck stiffness | 15 (78.95) | 9 (81.82 | 0 (0) |
| Final outcome | |||
| Died | 2 (10.53) | 0 (0) | 0 (0) |
Table 2.
Laboratory Findings in CSF Expressed as Means (±SD)
| Variable | BM (n = 19) | VM (n = 11) | Controls (n = 28) |
|---|---|---|---|
| WBC (count/μl) | 5,000 ± 1277.3 | 224.7 ± 90 | 6.32 ± 4.8 |
| Protein level (g/l) | 56.57 ± 5.89 | 36.8 ± 10.798 | 46.32 ± 8.044 |
| Glucose level (mg/dl) | 16.105 ± 3.626 | 50.45 ± 3.91 | 55.82 ± 3.302 |
| PCT (ng/ml) | 4.714 ± 1.59 | 0.1327 ± 0.03 | <0.1 |
| CRP | 0.884 ± 0.402 | <0.1 | <0.1 |
| hs‐CRP | 4.678 ± 1.25 | 0.9636 ± 0.8296 | 0.0789 ± 0.0339 |
WBC, white blood cell.
There was no gender difference detected, regarding PCT's concentration in CSF in all groups. The mean value of PCT was significantly higher in patients with BM in comparison to the control group and patients with VM (BM: 4.714 ± 1.59 ng/ml, VM: 0.1327 ± 0.03 ng/ml, control group: <0.1 ng/ml (0.0925 ± 0.02 ng/ml) with statistical significant difference between the BM group and the VM (P < 0.001), as well as between the BM and the control group (P < 0.001, Fig. 1). We also found that PCT's concentrations in CSF were related with age, in patients with BM, with significantly higher measurements in adult patients in comparison to children (mean 6.331 ± 2.74 vs. 3.196 ± 0.76 ng/ml, P = 0.0315; Fig. 2). Two patients from the study group with secondary hospital‐acquired BM, due to hospital multidrug‐resistant strains of A. baumannii (Table 3), who finally died, had even higher levels of PCT in comparison to patients with BM who survived (12.665 ± 2.1708 and 3.898 ± 1.766 ng/ml, P < 0.0001, respectively). PCT was detectable (0.2 ng/ml) in only three patients with VM.
Figure 1.
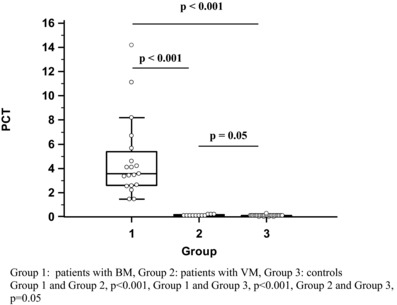
PCT levels in three groups.
Figure 2.
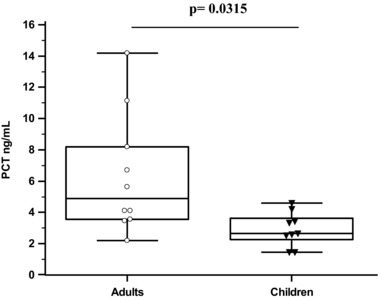
PCT levels in patients with BM in relation to their age.
Table 3.
Characteristics of Patients With Severe BM
| Patient 1 | Patient 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st day | 3rd day | 5th day | 6th day | 1st day | 3rd day | 5th day | 6th day | |
| Demographic data | ||||||||
| Age | 59 | 43 | ||||||
| Gender | Male | Male | ||||||
| CSF | ||||||||
| WBC (count/μl) | 360 | 4,500 | 5,000 | 4,800 | 300 | 1,560 | 2,100 | 2,200 |
| Protein level (g/l) | 46.8 | 62.3 | 48.5 | 52.3 | 41.2 | 45.1 | 43.4 | 42.8 |
| Glucose (mg/dl) | 42 | 6 | 8 | 10 | 48 | 20 | 18 | 22 |
| CRP | 0.5 | 3.1 | 4.1 | 3.5 | 0.1 | 0.4 | 0.5 | 0.41 |
| PCT (ng/ml) | 0.1 | 14.2 | 15.2 | 14.8 | 0.13 | 11.13 | 10.5 | 12.2 |
| Bacteria isolated | Negative | AB | AB | AB | Negative | AB | AB | AB |
AB, Acinetobacter baumannii.
The statistical analysis showed relatively high diagnostic value of PCT test in patients with BM. Its sensitivity was 100%, specificity 96.43%, PPV 95%, and NPV 100%. In group with VM, sensitivity was 27.27%, specificity 96.43%, PPV 75%, and NPV 77.14% (Table 4).
Table 4.
Sensitivity and Specificity of PCT Test in Cases With BM and VM
| Group | PCT+ | PCT− | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| BM (n = 19) | 19 | 0 | 100 | 96.43 | 95 | 100 |
| VM (n = 11) | 3 | 8 | 27.27 | 96.43 | 75 | 77.14 |
DISCUSSION
Meningitis is an infection of the membranes (meninges) that cover the brain and spinal cord, which can be due to bacterial, viral, or fungal infection. The diagnosis is based on clinical signs and laboratory CSF findings. In the emergency context, direct CSF examination provides evidence of BM in only 50 to 80% of cases 20. It is well known that other methods, which are used for the diagnosis, such as PCR, immunological, and biochemical tests have their own limitations. Furthermore, other protein biomarkers, such as pancreatic stone protein (PSP), soluble human triggering receptor expressed on myeloid cells 1 (sTREM‐1), macrophage migration inhibitory factor (MIF), are not yet established markers of infection. At present, only PCT is standardized and used in everyday clinical practice, while serum PCT is used in Europe for diagnosing BM as a routine biomarker 21, 22, 23. CSF's PCT test is not yet included in daily practice due to its cost and nonstandardization of cut‐off levels.
In this study, the PCT levels in CSF were significantly elevated in the BM group in comparison to VM and controls. The results are in agreement with other investigators who have evaluated different parameters such as WBC, CRP for the differential diagnosis of BM in patients with a negative direct CSF examination (Gram stain) 24, 25.
PCT is another marker that has been evaluated with regard to its usefulness in distinguishing between the possible causative organisms (bacterial or viral) for infections. Few published studies have focused on the value of PCT in CSF for the differential diagnosis of BM from VM. Jereb et al. confirmed the association of elevated PCT with bacterial infection. However, raised PCT in serum was also found in Hantavirus infections with overlapping results between viral and severe bacterial infections 26. Wacker et al. suggest that the results of the test must be interpreted with caution and in conjunction with medical history, physical examination, and microbiological data 27. The levels of PCT in this study were also correlated with age, something that is in agreement with findings by Ernst et al., who proposed that high PCT levels appear to be due to age or age‐related disorders of these subjects 16.
Additionally, PCT correlates with duration and severity of infection and has prognostic value for predicting the risk for mortality in critically ill patients with infections 28. Meng et al. reported that PCT ≥10 ng/ml had equivocal value with APACHE II score and CRP as a marker of short‐term mortality for critically ill patients 29. Our study revealed significant differences of the PCT concentration between survivors and deceased patients with BM. More specifically in deceased patients, the mean value of PCT in the CSF was 12.665 ± 2.1708 ng/ml. It should be studied more extensively and values ≥10 ng/ml should be evaluated for their significance in predicting mortality. To our knowledge, this is first study that questions the relationship between PCT in CSF and the final outcome of patients with meningitis.
PCT, apart from a specific marker of bacterial infection, can be considered in deciding which antibiotic is more appropriate to be used. Previous studies recommend the use of a PCT‐based algorithm for antibiotic therapy, in Intensive Care Unit patients with severe lower respiratory tract infections and sepsis 30. A recent systematic review and meta‐analysis of seven trials found a weighted mean antibiotic exposure reduction of 3.15 days (95% CI: 1.45−4.35) with a PCT‐based algorithm in comparison to non‐PCT‐based practice 31. Although studies have demonstrated that a PCT‐based antibiotic therapy protocol reduced antibiotic use in critically ill patients, unfortunately there are no studies considering the use of this kind of protocols for patients with BM. Measuring PCT in CSF gives additional information to the clinician and empowers any decision for initiating antibiotics, which are fundamental in the management of BM. In different postponement in delivering antibiotics can be detrimental for the patient and is associated with increased mortality. In this setting, a positive PCT result supports the clinical decision for the administration of antibiotics. Conversely, a negative PCT result removes the diagnostic thought from BM and subsequently the need for antibiotic treatment.
There is sufficient evidence to suggest the use of PCT in CSF for the diagnosis of BM. In conclusion, PCT can be a tool for the clinician, with the diagnostic enigma of BM and a prognostic marker of the disease's final outcome. Further studies with larger study groups need to be constructed in order to establish cut‐off levels for different ages of patients with BM.
CONFLICT OF INTEREST
There was not any potential conflict of interest in this article.
ACKNOWLEDGMENTS
We thank all the laboratory technicians and doctors from University General Hospital of Alexandroupolis who contributed to this study.
REFERENCES
- 1. Prasad R, Kapoor R, Mishra OP, Srivastava R, Kant Singh U. Serum procalcitonin in septic meningitis. Indian J Pediatr 2013;80(5):365–370. doi: 10.1007/s12098-012-0933-3. [DOI] [PubMed] [Google Scholar]
- 2. Alkholi UM, Al‐monem NA, El‐Azim AAA, Sultan MH. Serum procalcitonin in viral and bacterial meningitis. J Global Infect Dis 2011;3:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibrahim KA, Abdel‐Wahab AA, Ibrahim AS. Diagnostic value of serum procalcitonin levels in children with meningitis: A comparison with blood leukocyte count and C‐reactive protein. J Pak Med Assoc 2011;61:346–351. [PubMed] [Google Scholar]
- 4. Morgenthaler NG, Struck J, Chancerelle Y, et al. Production of procalcitonin (PCT) in non‐thyroidal tissue after LPS injection. Horm Metab Res 2003;35:290–295. [DOI] [PubMed] [Google Scholar]
- 5. Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin‐i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 2001;86:396–404. [DOI] [PubMed] [Google Scholar]
- 6. McCann FJ, Chapman SJ, Yu WC, et al. Ability of procalcitonin to discriminate infection from non‐infective inflammation using two pleural disease settings. PLoS One 2012;7(12):1–6, e49894. do.i:10.1371/journal.pone.0049894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bode‐Janisch S, Schutz S, Schmidt A, et al. Serum procalcitonin levels in the postmortem diagnosis of sepsis. Forensic Sci Int 2013;226:266–272. [DOI] [PubMed] [Google Scholar]
- 8. Meem M, Modak JK, Mortuza R, Morshed M, Shahidul Islam M, Saha SK. Biomarkers for diagnosis of neonatal infections: A systematic analysis of their potential as a point‐of‐care diagnostics. J Glob Health 2011;1(2):201–209. [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider HG, Lam QT. Procalcitonin for the clinical laboratory: A review. Pathology 2007;39(4):383–390. [DOI] [PubMed] [Google Scholar]
- 10. Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: A systematic review of the literature Crit Care 2006;10(5):R145. doi: 10.1186/cc5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jereb M, Muzlovic I, Hojker S, Strle F. Predictive value of serum and cerebrospinal fluid procalcitonin levels for the diagnosis of bacterial meningitis. Infection 2001;29(4):209–212. [DOI] [PubMed] [Google Scholar]
- 12. Mills GD, Lala HM, Oehley MR, et al. Elevated procalcitonin as a diagnostic marker in meningiococcal disease. Eur J Clin Microbiol Infect Dis 2006;25:501–509. [DOI] [PubMed] [Google Scholar]
- 13. Shimetani N, Shimetani K, Mori M. Levels of three inflammation markers, C‐reactive protein, serum amyloid A protein and procalcitonin, in the serum and cerebrospinal fluid of patients with meningitis. Scand J Clin Lab Invest 2001;61:567–574. [DOI] [PubMed] [Google Scholar]
- 14. Gendrel D, Raymond J, Assicot M, et al. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis 1997;24:1240–1242. [DOI] [PubMed] [Google Scholar]
- 15. Han YY, Carcillo JA, Ruppel RA, et al. Cerebrospinal fluid procalcitonin and severe traumatic brain injury in children. Pediatr Crit Care Med 2002;3:39–44. [DOI] [PubMed] [Google Scholar]
- 16. Ernst A, Morgenthaler NG, Buerger K, et al. Procalcitonin is elevated in the cerebrospinal fluid of patients with dementia and acute neuroinflammation. J Neuroimmunol 2007;189:169–174. [DOI] [PubMed] [Google Scholar]
- 17. de Ory F, Avellón A, Echevarría JE, et al. Viral infections of the central nervous system in Spain. A prospective study. J Med Virol 2013;85(3):554–562. doi: 10.1002/jmv.23470. [DOI] [PubMed] [Google Scholar]
- 18. Kupila L, Vuorinen T, Vainionpäā R, Marttila RJ, Kotilainen P. Diagnosis of enteroviral meningitis by use of polymerase chain reaction of cerebrospinal fluid, stool, and serum specimens Clin Infect Dis 2005;40(7):982–987. [DOI] [PubMed] [Google Scholar]
- 19. Soares CN, Cabral‐Castro MJ, Peralta JM, de Freitas MR, Zalis M, Puccioni‐Sohler M. Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. J Neurol Sci 2011;303(1‐2):75–79. [DOI] [PubMed] [Google Scholar]
- 20. Huy NT, Thao NT, Tuan NA, et al. Performance of thirteen clinical rules to distinguish bacterial and presumed viral meningitis in Vietnamese children. PLoS One 2012;7(11):1–8, e50341. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlapbach LJ, Graf R, Woerner A, et al. Pancreatic stone protein as a novel marker for neonatal sepsis. Intensive Care Med 2013;39(4):754–763. [DOI] [PubMed] [Google Scholar]
- 22. Su L, Han B, Liu C, et al. Value of soluble TREM‐1, procalcitonin, and C‐reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: A prospective cohort study. BMC Infect Dis 2012;12(157):1–10. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ostergaard C, Benfield T. Macrophage migration inhibitory factor in cerebrospinal fluid from patients with central nervous system infection. Crit Care 2009;13(3):1–8, R101. doi: 10.1186/cc7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spanos A, Harrell Fe Jr, Durack DT. Differential diagnosis of acute meningitis: An analysis of the predictive value of initial observations. JAMA 1989;262:2700–2707. [PubMed] [Google Scholar]
- 25. Ray P, Badarou‐Acossi G, Viallon A, Boutoille D, Arthaud M, Trystram D, Riou B: Accuracy of the cerebrospinal fluid results to differentiate bacterial from non bacterial meningitis, in case of negative gramstained smear. Am J Emerg Med 2007;25:179–184. [DOI] [PubMed] [Google Scholar]
- 26. Jereb M, Lunaček N, Kotar T, Saksida A, Petrovec M, Avšič‐Zupanc T. Procalcitonin in Hantavirus infections. Scand J Clinl Lab Invest 2011;71(4):287–291. [DOI] [PubMed] [Google Scholar]
- 27. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta‐analysis. Lancet Infect Dis 2013;13(5):426–435. doi: 10.1016/S1473-3099(12)70323-7. [DOI] [PubMed] [Google Scholar]
- 28. Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med 2006;34:2596–2602. [DOI] [PubMed] [Google Scholar]
- 29. Meng FS, Su L, Tang YQ, Wen Q, Liu YS, Liu ZF. Serum procalcitonin at the time of admission to the ICU as a predictor of short‐term mortality. Clin Biochem 2009;42:1025–1031. [DOI] [PubMed] [Google Scholar]
- 30. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010;375:463–474. [DOI] [PubMed] [Google Scholar]
- 31. Matthaiou DK, Ntani G, Kontogiorgi M, Poulakou G, Armaganidis A, Dimopoulos G. An ESICM systematic review and meta‐analysis of procalcitonin‐guided antibiotic therapy algorithms in adult critically ill patients. Intensive Care Med 2012;38:940–949. [DOI] [PubMed] [Google Scholar]


