Abstract
Background
The impact of being overweight remains unclear in Asian populations that tend to be lean. The objective of this study is to clarify the impact of body mass index (BMI) and metabolic factors on the prognosis of Japanese patients with IgA nephropathy (IgAN).
Methods
A total of 193 patients with IgAN were divided into three groups equally according to BMI: Group L (lean group, BMI: 15.6–20.1 kg/m2), Group M (middle group, BMI: 20.2–23.0 kg/m2), and Group O (obesity group, BMI: 23.1–31.9 kg/m2). Clinical data at the time of renal biopsy and the progression of the patients after renal biopsy were analyzed.
Results
At the time of renal biopsy, hypertension, dyslipidemia, hyperuricemia, and hypercomplementemia in Group O were more significant compared with those in Group L and/or Group M. Uric acid, triglyceride, C3, C4, high‐density lipoprotein cholesterol, serum creatinine, systolic blood pressure (BP), and diastolic BP were significantly correlated with BMI. In Group O, the remission of urinary protein over 5 years was significantly delayed using a log‐rank test. At the final observation, the BMI of each group was as similar as that at renal biopsy. The patients with aggressive therapy, such as steroid therapy and/or tonsillectomy in Group O did not have major side effects, except for a slight elevation of total cholesterol and low‐density lipoprotein cholesterol.
Conclusion
Even slightly high BMI seems to be a risk factor for progress in Japanese patients with IgAN.
Keywords: body mass index, IgA nephropathy, Japanese, metabolic syndrome, obesity
INTRODUCTION
IgA nephropathy (IgAN) is the most frequent chronic glomerulonephritis and its prognosis is poor: about 15 to 40% of patients will eventually have end‐stage kidney disease (ESKD) 1. Since IgAN is a chronic glomerular disease and progresses gradually over the long term, additional factors may have a lot of influence on the prognosis. Therefore, a more diversified treatment is needed.
Along with the development of metabolic syndrome analysis, more attention has been paid to the influence of obesity on chronic kidney disease (CKD). Bolignano et al. indicated that obesity is one of the independent risk factors for the development and progression of CKD 2. Similarly, excessive body mass index (BMI) is reported as a risk factor for disease progression in IgAN patients in several studies 3. On the other hand, there are ethnic differences in body size. In general, Asians are leaner than those of European origin. BMI cut‐off points for obesity are different in Asian countries from those that the World Health Organization (WHO) has defined. In comparison with the guideline issued by the WHO, where obesity has been defined as BMI ≥30 kg/m2 4, it is defined as ≥25 kg/m2 in Korea and Japan, ≥27 kg/m2 in Taiwan, and ≥28 kg/m2 in China 5. Moreover, Asians, including Japanese, are susceptible to the accumulation of visceral fat and are easy to develop type 2 diabetes and cardiovascular diseases, even with a BMI lower than 25 kg/m2 6, 7. Despite this ethnic difference of body size, in most of studies about obesity using BMI, the BMI cut‐off point was defined as 25 kg/m2. Since BMI of Asian patients with CKD is often below 25 kg/m2, the importance of appropriate body size tends to be underestimated.
The impact of overweight on the progression of IgAN among lean Asian individuals is not sufficiently elucidated. In this study, the authors analyzed the anthropometric data of 193 Japanese IgAN patients with a follow‐up period of more than 5 years to investigate the impact of high BMI on the progression of IgAN.
MATERIALS AND METHODS
Patients
All IgAN patients were diagnosed in the Juntendo University Hospital between January 1999 and May 2009. The study protocol was approved by the ethics committee of Juntendo University Hospital. Patients with diabetes mellitus or the autoimmune disease, purpura nephritis, were excluded from this study. There were 95 male (49.2%) and 98 female (50.8%) patients, with a median age of 32.8 ± 11.0 years (range: 12–65 years). In ascending order of BMI, the patients were divided equally into three groups: Group L (lean group; n = 65, BMI: 15.6–20.1 kg/m2), Group M (middle group; n = 64, BMI: 20.2–23.0 kg/m2), and Group O (obesity group; n = 64, BMI: 23.1–31.9 kg/m2). Clinical and pathological data at the time of renal biopsy were analyzed. Only two patients took lipid‐lowering agents at the time of biopsy.
Treatment Protocol
For steroid pulse therapy, patients received intravenous methylprednisolone pulse therapy of 0.5 g/day for three consecutive days, followed by oral prednisolone at an initial dosage of 0.5 mg/kg every other day for 6 months. The oral prednisolone was gradually tapered off every 2 months, as follows: 0.25 mg/kg, 0.125 mg/kg, 0.0625 mg/kg, and off. The definition of “aggressive therapy” is steroid therapy, including oral steroid and/or steroid pulse, and/or tonsillectomy 8.
Biochemical Assessment (Clinical Data)
All samples collected were from fasting subjects and were analyzed at the time of biopsy and the final observation at a single center (Juntendo University Hospital). BMI was calculated as weight in kilograms divided by the square of height in meters. Biochemical analysis, including the measurement of hemoglobin (Hb), hematocrit (Hct), total protein (TP), albumin (Alb), serum urea nitrogen (SUN), serum creatinine (s‐Cr), uric acid (UA), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐c), high‐density lipoprotein cholesterol (HDL‐c), triglyceride (TG), IgG, IgA, IgM, C3, C4, CH50, C‐reactive protein (CRP) levels, and urinary protein (UP) was performed using standard enzymatic methods. The estimated glomerular filtration rate (eGFR) was calculated using the formula established by the Japanese Society of Nephrology as follows: eGFR (ml/min/1.73m2) = 194 × Cr−1.094 × age −0.287 (if female, × 0.739) 9. Fasting plasma glucose (FPG) and HbA1c were also measured at the final observation.
The degree of red blood cells (uRBCs) in the urinary sediments was scored into five degrees as follows: 1–5 RBCs/high‐power field (HPF), 1; 6−10 RBCs/HPF, 2; 11–15 RBCs/HPF, 3; 16–20 RBCs/HPF, 4; and more than 21 RBCs/HPF, 5 10. UP and urinary creatinine were measured using a spot urine analysis to gain the UP to creatinine ratio. The median follow‐up time after renal biopsy was 5 years. The levels of s‐Cr, UP, and uRBCs after the renal biopsy were yearly compared after 5 years. We defined UP <0.3 g/gCr as UP remission, uRBCs <5 /HPF as uRBCs remission, and both UP <0.3 g/gCr and uRBCs <5/HPF as complete remission 8.
Morphometric Analysis
Paraffin‐embedded sections were routinely stained with hematoxylin and eosin, periodic acid–Schiff, Masson's trichrome, and periodic acid–silver methenamine. The activity and severity were graded using the Japanese classification of IgAN 11. Staining for IgG, IgA, IgM, C3c, and C1q on freshly frozen renal tissue was performed using a corresponding fluorescence isothiocyanate‐conjugated anti‐sera (Dako, Copenhagen, Denmark). Renal tissues were immediately fixed in a 2% osmium and phosphate buffer saline for 2 hr, dehydrogenated by increasing the concentration of alcohol, and then embedded in Epok 812 (Oken‐syoji, Tokyo, Japan).
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) of the mean or as percentages. Normality of each continuous variable was initially examined using the Bartlett's test. The clinical parameters of the three groups were compared using an analysis of variance (ANOVA) with Bonferroni's multiple comparison test for normally distributed continuous variables or Kruskal–Wallis test with post hoc for nonnormally distributed variables. Categorical variables were also compared using a Chi‐square test or Fisher's exact test. A Spearman's correlation coefficient by rank was performed to examine the relationship between BMI and clinical data. The cumulative probability of remission of UP and uRBCs was compared using the log‐rank test. The multivariate Cox regression analysis was used to assess the determinants of remission of proteinuria. Statistical significance was considered as P < 0.05. All statistical analyses were performed by using GraphPad Prism 6, version 6.0c (GraphPad, San Diego, CA) or the JMP 9.0.1 (SAS Institute Inc., Cary, NC) software program.
RESULTS
Distribution of BMI and Age
The distribution peak of BMI shifted to the left. The number of patients whose BMI was greater than 25 was only 15% of the total subjects (Fig. 1). The bars of the figure are divided by age categories. Those who have a small BMI tended to be young.
Figure 1.
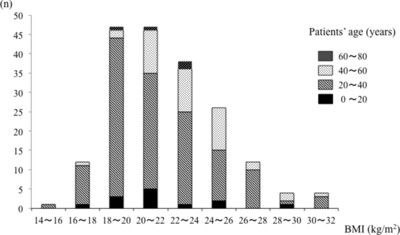
Distribution of BMI of patients with IgAN.
Comparison of Clinical Parameters at the Time of Renal Biopsy
Table 1 shows the baseline characteristics of the three groups. The mean of baseline BMI (n = 193) was 21.9 ± 3.1 kg/m2. When we divided the patients into three groups according to BMI, the cut‐off points were 20.1 kg/m2 between Group L and Group M and 23.0 kg/m2 between Group M and Group O, respectively. The ratio of females to males was significantly higher in Group L, even in Group M, and lower in Group O. Systolic blood pressure (sBP) and diastolic BP (dBP) in Group O were significantly more elevated than those in Group L and Group M. eGFR in Group L was higher than that in Group O. Levels of TG, LDL‐C, UA, Hb, CRP, C3, and C4 in Group O were greater than those in Groups L and M. SUN, TP, Alb, IgG, IgM, IgA, UP, and uRBCs did not differ among the three groups. As for the differences between Group L and Group M, there were also significant difference between the groups in age, blood pressure, and TG.
Table 1.
Clinical Background of All Patients With IgAN
| Group L (n = 65) | Group M (n = 64) | Group O (n = 64) | P | |
|---|---|---|---|---|
| Anthropometric variables | ||||
| Gender (m:f) | 18:47 | 31:33 | 46:18 | <0.01a |
| Age (years) | 28.5 ± 8.1 | 33.7 ± 11.8 | 36.3 ± 11.5 | <0.01 L vs. M, L vs. O |
| BMI (kg/m2) | 18.8 ± 1.1 | 21.5 ± 0.9 | 25.5 ± 2.0 | <0.01 L vs. M, L vs. O, M vs. O |
| sBP (mmHg) | 110.1 ± 13.0 | 115.8 ± 12.0 | 120.8 ± 14.0 | <0.01 L vs. M, L vs. O |
| dBP (mmHg) | 64.3 ± 9.7 | 65.6 ± 8.7 | 71.6 ± 12.3 | <0.01 L vs. M, M vs. O |
| Biochemical variables | ||||
| s‐Cr (mg/dl) | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.9 ± 0.3 | <0.01 L vs. M, L vs. O |
| eGFR (ml/min/1.73 m2) | 93.0 ± 23.1 | 84.2 ± 26.8 | 77.9 ± 22.3 | <0.01 L vs. O |
| uPro (g/g creatinine) | 1.0± 1.3 | 1.2 ± 1.8 | 0.9 ± 1.0 | NS |
| uRBC (grade) | 4.4 ± 1.2 | 4.1 ± 1.4 | 3.9 ± 1.5 | NS |
| SUN (mg/dl) | 14.0 ± 4.6 | 14.9 ± 4.5 | 15.1 ± 3.8 | NS |
| UA (mg/dl) | 5.5 ± 1.6 | 5.0 ± 1.3 | 6.6 ± 1.5 | <0.01 L vs. O, M vs. O |
| TP (g/dl) | 6.7 ± 0.6 | 6.8 ± 0.6 | 6.9 ± 0.6 | NS |
| Alb (g/dl) | 4.1 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.4 | NS |
| TC (mg/dl) | 189.0 ± 35.3 | 197.1 ± 36.9 | 211.1 ± 41.4 | <0.01 L vs. O |
| TG (mg/dl) | 81.1 ± 35.6 | 115.1 ± 58.1 | 150.0 ± 87.1 | <0.01 L vs. M, L vs. O, M vs. O |
| HDL‐c (mg/dl) | 67.0 ± 13.8 | 68.4 ± 24.9 | 56.1 ± 20.1 | <0.01 L vs. O, M vs. O |
| LDL‐c (mg/dl) | 107.9 ± 28.9 | 111.6 ± 29.6 | 126.2 ± 34.7 | <0.01 L vs. O, M vs. O |
| Hb (g/dl) | 12.9 ± 1.4 | 13.2 ± 1.4 | 14.2 ± 1.4 | <0.01 L vs. O, M vs. O |
| Immunological variables | ||||
| CRP (mg/dl) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.4 | <0.01 L vs. O |
| IgG (mg/dl) | 1214.9 ± 317.6 | 1124.1 ± 274.5 | 1125.5 ± 247.1 | NS |
| IgA (mg/dl) | 312.1 ± 89.7 | 317.4 ± 105.8 | 316.9 ± 99.9 | NS |
| IgM (mg/dl) | 137.3 ± 60.0 | 128.6 ± 69.6 | 112.7 ± 58.8 | NS |
| C3 (mg/dl) | 92.2 ± 13.6 | 96.4 ± 14.0 | 107.8 ± 18.8 | <0.01 L vs. O, M vs. O |
| C4 (mg/dl) | 20.9 ± 4.9 | 24.2 ± 7.5 | 27.6 ± 6.5 | <0.01 L vs. M, L vs. O, M vs. O |
| CH50 (unit/ml) | 39.2 ± 6.3 | 41.3 ± 7.0 | 43.9 ± 7.4 | <0.01 L vs. O |
Data compared using Fisher's exact test.
Values are expressed as percent or mean ± SD.
NS, not significant.
Correlation Between BMI and Clinical Variables in All Patients
BMI was significantly correlated with UA, TG, C4, Hct, Hb, C3, HDL‐c, s‐Cr, sBP, dBP, CRP, eGFR, LDL‐c, age, CH50, TC, and SUN (Table 2).
Table 2.
Relationship of BMI and Clinical Data by Linear Regression Models
| R | P | R | P | ||
|---|---|---|---|---|---|
| UA (mg/dl) | 0.44270 | <0.0001 | CRP (mg/dl) | 0.26390 | <0.0001 |
| TG (mg/dl) | 0.42310 | <0.0001 | eGFR (ml/min/1.73m2) | −0.25160 | 0.0004 |
| C4 (mg/dl) | 0.41790 | <0.0001 | LDL‐c (mg/dl) | 0.25150 | 0.0009 |
| Hct (%) | 0.36060 | <0.0001 | Age (years) | 0.25010 | 0.0005 |
| Hb (g/dl) | 0.35400 | <0.0001 | CH50 (Unit/ml) | 0.24500 | 0.0010 |
| C3 (mg/dl) | 0.35750 | <0.0001 | TC (mg/dl) | 0.20580 | 0.0047 |
| HDL‐c (mg/dl) | −0.34800 | <0.0001 | SUN (mg/dl) | 0.15640 | 0.0298 |
| s‐Cr (mg/dl) | 0.34160 | <0.0001 | Alb (mg/dl) | 0.08178 | NS |
| sBP (mmHg) | 0.33110 | <0.0001 | TP (g/dl) | 0.03926 | NS |
| dBP (mmHg) | 0.30520 | <0.0001 | UP (g/g creatinine) | 0.03182 | NS |
NS, not significant; R, regression.
Pathological Findings
In the light microscopic findings, there were no significant differences among the three groups (Table 3), though Group M and Group O tended to have arteriosclerotic findings. In the immunofluorescence findings, there were fewer patients with depositions of IgA, IgG, and IgM in Group O.
Table 3.
Pathological Background of all Patients With IgAN
| Group L | Group M | Group O | P | |
|---|---|---|---|---|
| Light microscopic findings | ||||
| Total glomeruli (n) | 17.6 ± 7.73 | 16.75 ± 8.36 | 14.24 ± 7.19 | <0.05a |
| Histologic gradec(I:II:III:IV; n) | 48:12:4:1 | 35:17:11:1 | 38:17:9:0 | NSb |
| Number of global sclerosis | 1.6 ± 1.9 | 2.0 ± 2.6 | 1.9 ± 2.4 | NSa |
| Number of segmental sclerosis | 0.4 ± 0.8 | 0.9 ± 2.0 | 0.7 ± 1.3 | NSa |
| Number of crescent | 1.4 ± 2.0 | 1.7 ± 2.6 | 1.0 ± 1.3 | NSa |
| Double contour (n) | 17 | 16 | 12 | NSa |
| Lobulation (n) | 7 | 6 | 2 | NSa |
| Arteriole sclerosis (n) | 15 | 24 | 23 | NSa |
| Tubular atrophy score (0:1:2:3:4:5) | 40:8:0:0:1 | 43:8:0:1:3 | 45:9:0:0:0 | NSb |
| Immunofluorescence findings | ||||
| IgA, n (%) | 32 (37.6) | 25 (29.4) | 28 (32.9) | NSb |
| IgA + IgM, n (%) | 8 (24.2) | 13 (39.4) | 12 (36.4) | NSb |
| IgA + IgG, n (%) | 15 (39.5) | 8 (21.1) | 15 (39.5) | NSb |
| IgA + IgM + IgG, n (%) | 10 (27.0) | 19 (51.4) | 8 (21.6) | <0.05b |
Data compared using one‐way factorial ANOVA.
Data compared using Fisher's exact test.
Japanese classification of IgAN.
NS, not significant.
Treatment
Several treatment strategies were chosen during the follow‐up period, and there was a significant difference (Table 4). In Group O, tonsillectomy and steroid pulse was performed less frequently. Renin–angiotensin system inhibitors and calcium channel blockers were prescribed more frequently.
Table 4.
Treatment
| Treatment | Group L | Group M | Group O | P |
|---|---|---|---|---|
| Ton, n (%) | 20 (30.8) | 19 (29.7) | 10 (15.6) | NS |
| Ton + SP + SO, n (%) | 19 (29.2) | 16 (25.0) | 7 (10.9) | <0.01 |
| SP + SO, n (%) | 30 (46.2) | 18 (28.1) | 12 (18.8) | <0.01 |
| SO, n (%) | 1 (1.5) | 4 (6.3) | 2 (3.1) | NS |
| RAS‐I, n (%) | 16 (24.6) | 22 (34.4) | 32 (50.0) | <0.01 |
| CCB, n (%) | 0 (0) | 7 (10.9) | 13 (20.3) | <0.01 |
Data compared using Fisher's exact test.
NS, not significant; Ton, tonsillectomy; SP, steroid pulse therapy; SO, steroid oral prescription; RAS‐I, renin–angiotensin system inhibitor; CCB, calcium channel blocker.
Comparison of the Incidence Rate of Remission of UP and uRBCs
The remission of UP and uRBCs was compared among the three groups. Because the change of eGFR was scarce during the observation period (data not shown), the remission rate of uRBCs and UP was compared. Remission of UP was significantly delayed in Group O over 5 years (Fig. 2). Especially in the first 10 months, the UP of approximately half of the patients in Group M and Group L disappeared. On the other hand, only 20% of the patients in Group O showed a UP remission. In order to ascertain whether the low remission rate of the patients in Group O was because fewer patients were given aggressive therapy, the three groups were divided into six groups by the presence or absence of aggressive therapy (Fig. 3). In patients with aggressive therapy, UP remission was similar among the three groups. With aggressive therapy, UP in about half of the patients in each group disappeared within only 10 months. In patients without aggressive therapy, UP remission in Group O was significantly delayed.
Figure 2.
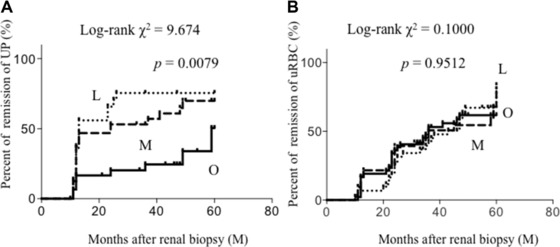
Cumulative probability of remission of (A) UP and (B) uRBCs. Percentage of remission of UP in Group O was significantly lower than those in other groups. L, lean group; M: middle group; O, obesity group.
Figure 3.
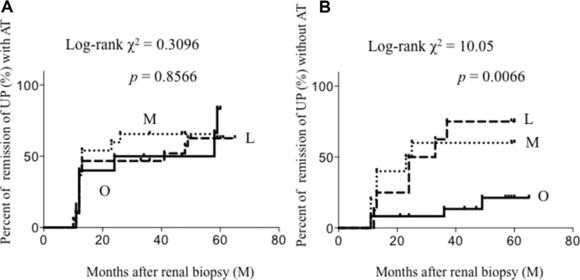
Cumulative probability of remission of UP (A) with aggressive therapy, (B) without aggressive therapy. Percentage of remission of UP in Group O without aggressive therapy was significantly lower than those in other groups. L, lean group; M, middle group; O, obesity group; AT, aggressive therapy.
Comparison of the Clinical Findings at the Final Observation
In order to evaluate not only urinary findings and renal function, but also the change of body size and clinical data, we collected the clinical data and BMI of patients at their last follow‐up period. Consequently, 80 of the 193 patients were able to have their BMI checked at the final observation. The data of all patients treated at our faculty with a renal biopsy were not collected because there were patients who dropped out from the outpatient clinic because of urinary remission or because of a move. There were two patients who needed renal replacement therapy (RRT) among the patients in Group M and one patient in Group L during the observation period. All of them had not been given aggressive therapy (data not shown).
In the patients without aggressive therapy in Group O, BMI, TG, UA, and systolic BP were higher, and HDL‐c was lower (Table 5). On the other hand, in the patients with aggressive therapy in Group O, TC and LDL‐c were a little higher than the other groups. There were no patients who developed diabetes, cataract, bone abnormalities during the observation period and their FPG and HbA1c were similar to those at renal biopsy. In all groups, BMI had not changed significantly during the observation period. s‐Cr in patients without aggressive therapy in Group M was higher than that in the other groups.
Table 5.
Comparison of Clinical Findings at Final Observation
| Group L | Group M | Group O | P | |||||
|---|---|---|---|---|---|---|---|---|
| Aggressive Therapy | + | − | + | − | + | − | ||
| n | 17 | 8 | 17 | 9 | 8 | 21 | ||
| Gender (m:f) | 6:11 | 3:5 | 4:13 | 5:4 | 4:4 | 17:4 | <0.05 | |
| Observation period | 89.1 ± 25.0 | 82.5 ± 36.9 | 78.8 ± 31.8* | 129.3 ± 31.3† | 94.3 ± 31.4 | 106.3 ± 35.1 | <0.01 | * vs. †, P < 0.01 |
| Age (years) | 35.4 ± 8.2 | 42.4 ± 13.8 | 40.7 ± 9.1 | 47.3 ± 11.4 | 37.6 ± 5.9 | 49.9 ± 11.6 | <0.01 | |
| BMI (kg/m2) | 19.5 ± 1.3* | 20.0 ± 4.2‡ | 20.9 ± 1.6 | 21.2 ± 1.9 | 23.8 ± 3.8† | 25.4 ± 3.1§ | <0.01 | * versus †, P < 0.01, ‡ versus §, P < 0.01 |
| sBP (mmHg) | 109.7 ± 9.7 | 105.3 ± 13.3* | 113.8 ± 14.9 | 118.9 ± 8.3 | 117.3 ± 15.5 | 121.3 ± 10.0† | <0.01 | * versus †, P < 0.05 |
| dBP (mmHg) | 69.2 ± 9.2 | 68.0 ± 8.4 | 68.9 ± 10.8 | 69.8 ± 7.7 | 73.3 ± 11.3 | 76.1 ± 9.6 | NS | |
| s‐Cr (mg/dl) | 0.78 ± 0.33 | 0.66 ± 0.24* | 0.93 ± 0.55 | 1.52 ± 1.59 | 0.84 ± 0.23 | 1.07 ± 0.57† | <0.05 | * versus †, P < 0.05 |
| eGFR (ml/min/1.73 m2) | 84.3 ± 23.4 | 92.4 ± 22.8 | 73.4 ± 30.9 | 67.5 ± 35.2 | 76.3 ± 17.2 | 67.9 ± 24.9 | NS | |
| uPro (g/g creatinine) | 0.22 ± 0.31 | 0.13 ± 0.19 | 0.18 ± 0.25 | 0.61 ± 0.75 | 0.26 ± 0.27 | 0.55 ± 0.78 | NS | |
| uRBCs (grade) | 1.18 ± 0.53 | 2.00 ± 1.53 | 1.44 ± 1.09 | 1.22 ± 0.44 | 1.00 ± 0.00 | 1.76 ± 0.94 | NS | |
| UA (mg/dl) | 4.8 ± 0.8 | 4.4 ± 0.8* | 5.1 ± 1.5 | 5.5 ± 1.7 | 5.9 ± 1.3 | 6.4 ± 1.1† | <0.01 | * versus †, P < 0.01 |
| Alb (g/dl) | 4.3 ± 0.29 | 4.24 ± 0.26 | 4.25 ± 0.27 | 4.08 ± 0.34 | 4.21 ± 0.13 | 4.12 ± 0.37 | NS | |
| TC (mg/dl) | 194.0 ± 31.1 | 197.6 ± 20.8 | 182.6 ± 32.8 | 184.3 ± 36.9 | 214.0 ± 42.9 | 199.5 ± 24.8 | NS | |
| TG (mg/dl) | 89.9 ± 31.8 | 86.8 ± 32.5* | 108.7 ± 49.2 | 132.6 ± 72.9 | 105.9 ± 35.9 | 162.8 ± 68.1† | <0.01 | * versus †, P < 0.05 |
| HDL‐c (mg/dl) | 70.9 ± 13.8 | 69.5 ± 6.9* | 63.1 ± 18.9 | 58.1 ± 23.8 | 65.0 ± 25.3 | 48.0 ± 9.8† | <0.01 | * versus †, P < 0.05 |
| LDL‐c (mg/dl) | 102.1 ± 22.1 | 110.8 ± 22.2 | 99.6 ± 22.6 | 97.5 ± 24.4 | 127.8 ± 28.6 | 119.1 ± 22.4 | <0.05 | |
| CRP (mg/dl) | 0.07 ± 0.13 | 0.05 ± 0.12 | 0.09 ± 0.09 | 0.03 ± 0.05 | 0.07 ± 0.05 | 0.12 ± 0.09 | NS | |
| IgA (mg/dl) | 239.3 ± 74.9 | 281.7 ± 86.6 | 297.6 ± 106.6 | 270.5 ± 72.6 | 223.5 ± 77.4 | 315.7 ± 106.4 | NS | |
| C3 (mg/dl) | 89.5 ± 10.5 | 84.0 ± 11.0 | 89.5 ± 12.2 | 84.7 ± 14.1 | 92.0 ± 23.6 | 101.3 ± 18.0 | NS | |
| FPG (mg/dl) | 94.7 ± 28.4 | 86.6 ± 9.5 | 88.6 ± 11.0 | 83.8 ± 6.7 | 83.1 ± 8.0 | 99.1 ± 20.9 | NS | |
| HbA1c (%) | 5.1 ± 0.2 | 5.5 ± 0.1 | 5.2 ± 0.3 | 5.2 ± 0.4 | 5.4 ± 0.3 | 5.5 ± 0.4 | NS | |
Values are expressed as percent or mean ± SD.
NS, not significant.
DISCUSSION
The most important finding of this study was that in IgAN, patients with high BMI achieved UP remission slower than other groups and that might lead to a worsening of their prognosis. In the patients without aggressive therapy, the differences among the three groups were more evident, whereas with aggressive therapy, the differences disappeared. These results indicate that in IgAN high BMI is an important factor that interferes in the remission of proteinuria, especially in the absence of aggressive treatment. Thus, we should not leave patients with high BMI untreated.
In this study, “aggressive therapy” includes steroid oral administration, steroid pulse therapy, tonsillectomy, or a combination of them. Pozzi reported the effectiveness and safety of steroid pulse therapy 12. A combination of tonsillectomy and steroid pulse therapy was developed in Japan 13. In Japan, UP >0.5 g/day and eGFR >60 ml/min/1.73m2 is a provisionary criterion for tonsillectomy combined with steroid pulse therapy 14, but the adequate adaptation of these aggressive therapies has not been clearly defined. In our study, we observed that the patients with aggressive therapy in Group O did not have any major side effects. Therefore, the result suggests that the adaptation of aggressive therapy on patients with obesity should be extended.
Three mechanisms are indicated as influences of overweight on the progression of IgAN. First, patients with overweight have metabolic‐related disorders like dyslipidemia, hyperuricemia, and hypertension, which lead to insulin‐resistance and finally, accelerated arteriosclerosis. In this study, metabolic parameters were much worse in the patients with higher BMI and showed a significant correlation with BMI. Each of them is also pointed out to have an effect on the progress of IgAN patients 15, 16, 17, 18. Second, when overweight accompanies IgAN, pathological changes may have an effect on the prognosis. Tanaka et al. 19 reported that nondiabetic obese patients with IgAN showed significantly increased proteinuria, accompanied by glomerular basement membrane thickening and glomerulomegaly, like obesity‐related glomerulopathy 20. Third, recent studies indicate that overweight itself likely has effects on renal injury through various hemodynamic and nonhemodynamic mechanisms of adipocytes.
As to the third mechanism, recently, adipocytes have been revealed to be not only storage cells but also the production site for a variety of hormones and proinflammatory molecules that may contribute to progressive renal damage 21. Metabolic disorders are well known to be associated with inflammation 22. Although leptin is famous as an adipocytokine, the degeneration product of C3 is also considered to be one type of adipocytokine. In this study, the concentration of serum C3 in Group O was significantly higher than that in the other groups, although they remained within the normal limit. We also found a slight elevation of CRP, which is considered to reflect chronic, low‐grade inflammation due to overweight. A complement cascade is activated within the sites of connective tissues and atherosclerotic lesions 23. The authors have previously demonstrated that male patients with metabolic syndrome showed higher concentrations of serum C3, and C3 is closely related to insulin resistance 24. In IgAN patients, we also have indicated that the serum C3 level is closely related to nutritional status, metabolic syndrome, and the prognosis of the patients 25. Kim et al. 26 reported that a low level of serum C3 and mesangial C3 deposition were independent risk factors for poor renal outcome in patients with IgAN. In this study, high BMI had a strong relation with inflammation and the degeneration product of C3 may also correlate with BMI as one type of adipocytokine through artherosclerosis. We need further analysis to clarify the association of serum C3 and artherogenic change.
Body size has racial difference. In this study, we did not define the cut‐off point for obesity and divided them equally into three groups according to order of BMI. Consequently, the border between Group M and Group O became 23. Our results implied that IgAN might progress faster in Japanese patients with a BMI between 23 and BMI 25 because of overweight. Similarly, there is increasing evidence of an emerging high prevalence of type 2 diabetes and increased cardiovascular risk factors in parts of Asian countries where the average BMI is below the cut‐off point of 25 kg/m2 6, 7. Asians generally have a higher percentage of body fat than white people of the same age, sex, and BMI 27. Therefore, when we evaluate Asian patients with renal disease, defining a smaller BMI cut‐off point for obesity may be better. Although we were not able to show the ideal BMI in this study, our results indicated that patients with IgAN should target a smaller BMI in order to improve the prognosis of IgAN.
Several researchers reported that in elderly and non‐Caucasian populations, the relationship between BMI and body fatness might be different as compared with a younger Caucasian population 28. Despite the patients in our study being Japanese, their metabolic factors were significantly correlated with BMI. We consider that the reason is that the individuals in our study were comparably young. In our study, there were young patients with high BMI. As some researchers have noted that being overweight in early adulthood is strongly associated with declined kidney function in later life 29, 30, reducing weight may be important especially for young patients with obesity.
This study has several limitations. First, waist circumference (WC) was not measured. Recently, WC and the waist to hip ratio (WHR), which can estimate abdominal fat more precisely, are preferably used. It would be advisable to check not only BMI but also the WC and WHR of patients with IgAN regularly in the future. Second, there were patients who dropped out from our outpatient clinic. Some patients moved and others might suffer ESKD and underwent renal RRT. Third, this study is a retrospective study and we could not control the selection bias for treatment.
In conclusion, in Japanese patients with IgAN, even slightly high BMI seems to be a risk factor for progress. They should reduce their body weight and improve their metabolic factors.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest (COI) exists.
ACKNOWLEDGMENTS
We thank Ms. Takako Ikegami and Tomomi Ikeda, Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan, for their technical assistance. We also thank Ms. Akie Toki for her excellent technical assistance.
REFERENCES
- 1. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med 2002;347:738–748. [DOI] [PubMed] [Google Scholar]
- 2. Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: A systematic review. Nephrol Dial Transplant 2013;28(Suppl 4):iv82–iv98 [DOI] [PubMed] [Google Scholar]
- 3. Bonnet F, Deprele C, Sassolas A, et al. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis 2001;37:720–727. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1–253. [PubMed] [Google Scholar]
- 5. Bei‐Fan Z. Cooperative Meta‐Analysis Group of Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: Study on optimal cut‐off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr 2002;11(Suppl 8):S685–S693. [PubMed] [Google Scholar]
- 6. Huxley R, James WP, Barzi F, et al. Obesity in Asia Collaboration. Ethnic comparisons of the cross‐sectional relationships between measures of body size with diabetes and hypertension. Obes Rev 2008;9(Suppl 1):53–61. [DOI] [PubMed] [Google Scholar]
- 7. Low S, Chin MC, Ma S, et al. Rationale for redefining obesity in Asians. Ann Acad Med Singapore 2009;38:66–69. [PubMed] [Google Scholar]
- 8. Matsuzaki K, Suzuki Y, Nakata J, et al. Nationwide survey on current treatments for IgA nephropathy in Japan. Clin Exp Nephrol 2013;17:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuzawa Y. Metabolic syndrome—Definition and diagnostic criteria in Japan. J Atheroscler Thromb 2005;12:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Nakayama K, Ohsawa I, Maeda‐Ohtani A, et al. Prediction of diagnosis of immunoglobulin A nephropathy prior to renal biopsy and correlation with urinary sediment findings and prognostic grading. J Clin Lab Anal 2008;22:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomino Y, Sakai H. Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol 2003;7:93–97. [DOI] [PubMed] [Google Scholar]
- 12. Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: Long‐term results of a randomized, controlled trial. F. J Am Soc Nephrol 2004;15:157–163. [DOI] [PubMed] [Google Scholar]
- 13. Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 2001;38:736–743. [DOI] [PubMed] [Google Scholar]
- 14. Ohsawa I, Kusaba G, Ishii M, et al. Extraglomerular C3 deposition and metabolic impacts in patients with IgA nephropathy. Nephrol Dial Transplant 2013;28:1856–1864. [DOI] [PubMed] [Google Scholar]
- 15. Ohno I, Hosoya T, Gomi H, et al. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron 2001;87:333–339. [DOI] [PubMed] [Google Scholar]
- 16. Wakai K, Kawamura T, Endoh M, et al. A scoring system to predict renal outcome in IgA nephropathy: From a nationwide prospective study. Nephrol Dial Transplant 2006;21:2800–2808. [DOI] [PubMed] [Google Scholar]
- 17. Syrjänen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 2000;15:34–42. [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Zhang Y, Chen S, et al. Association of metabolic syndrome and IgA nephropathy. J Clin Pathol 2010;63:697–701. [DOI] [PubMed] [Google Scholar]
- 19. Tanaka M, Yamada S, Iwasaki Y, et al. Impact of obesity on IgA nephropathy: Comparative ultrastructural study between obese and non‐obese patients. Nephron Clin Pract 2009;112:c71–c78. [DOI] [PubMed] [Google Scholar]
- 20. Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity‐related glomerulopathy: An emerging epidemic. Kidney Int 2001; 59:1498–1509. [DOI] [PubMed] [Google Scholar]
- 21. Suganami T, Ogawa Y. Adipose tissue macrophages: Their role in adipose tissue remodeling. J Leukoc Biol 2010;88:33–39. [DOI] [PubMed] [Google Scholar]
- 22. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860–867. [DOI] [PubMed] [Google Scholar]
- 23. Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res 2004;30:73–80. [DOI] [PubMed] [Google Scholar]
- 24. Ohsawa I, Inoshita H, Ishii M, et al. Metabolic impact on serum levels of complement component 3 in Japanese patients. J Clin Lab Anal 2010;24:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki H, Ohsawa I, Kodama F, et al. Fluctuation of serum C3 levels reflects disease activity and metabolic background in patients with IgA nephropathy. J Nephrol 2013;26:708–715. [DOI] [PubMed] [Google Scholar]
- 26. Kim SJ, Koo HM, Lim BJ, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One 2012;7:e40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 28. Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006;35:83–92. [DOI] [PubMed] [Google Scholar]
- 29. Silverwood RJ, Pierce M, Thomas C, et al.; National Survey of Health and Development Scientific and Data Collection Teams. Association between younger age when first overweight and increased risk for CKD. J Am Soc Nephrol 2013;24:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non‐diabetic chronic kidney disease: A retrospective cohort study. Nephron Clin Pract 2009;113:c16–c23. [DOI] [PubMed] [Google Scholar]


