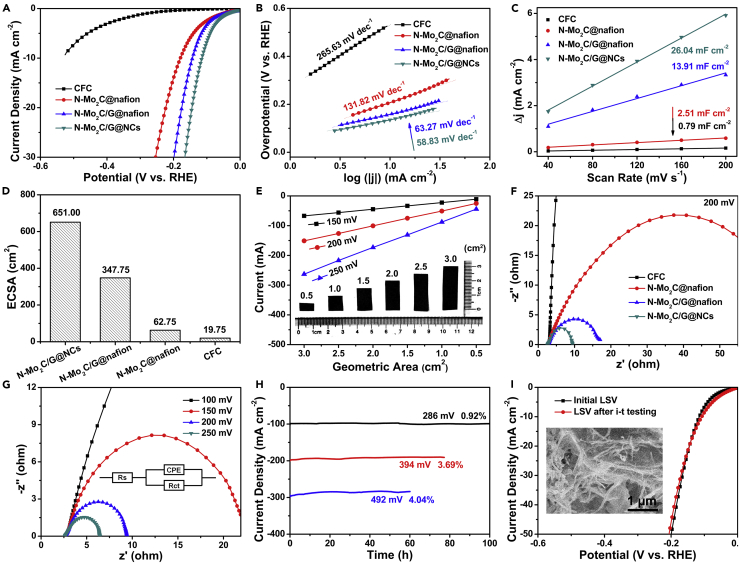
Figure 4.
HER Performance of Bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs
(A and B) (A) The LSV curves (iR uncorrected) and (B) the corresponding Tafel plots of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs.
(C and D) (C) Plot of positive and negative current density differences at a given potential (0.15 V versus RHE) against the CV scan rates, and (D) electrochemical active surface area (ECSA) of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs electrodes.
(E) A plot of current for HER as a function of the geometric area from N-Mo2C/G@NCs film under different overpotentials. The inset of (E) was the photos of N-Mo2C/G@NCs with different geometric area.
(F) EIS Nyquist plots at −200 mV versus RHE recorded of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs electrodes.
(G) EIS Nyquist plots of N-Mo2C/G@NCs with different overpotentials.
(H) The chronoamperometry curves of the N-Mo2C/G@NCs electrode recorded at the overpotentials of 286, 394, and 492 mV.
(I) The LSV curves of N-Mo2C/G@NCs electrode after i-t testing. The inset of (I) displayed the corresponding SEM image after i-t testing. All tests were performed in 0.5 M H2SO4 solution.
See also Figures S25–S30.