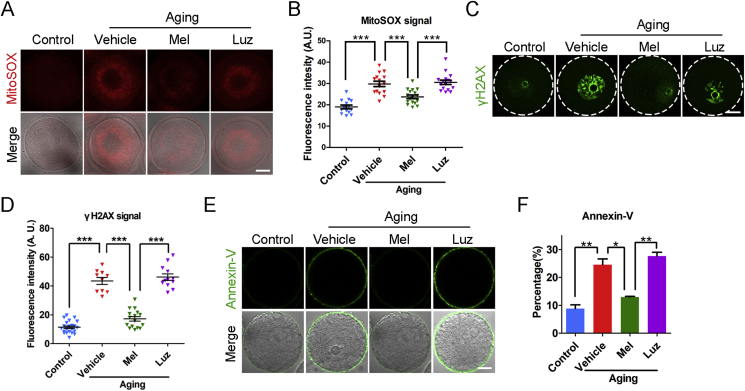
Fig. 3.
Effects of melatonin administration in vivo on the ROS levels and apoptosis of aged oocytes. (A) Representative images of ROS levels stained with MitoSOX in young, aged, melatonin-administered and melatonin + luzindole-administered oocytes. Scale bar, 20 μm. (B) Fluorescence intensity of MitoSOX signals was recorded in young (n = 14), aged (n = 15), melatonin-administered (n = 17) and melatonin + luzindole-administered (n = 16) oocytes. (C) Representative images of DNA damage stained with γH2AX antibody in young, aged, melatonin-administered and melatonin + luzindole-administered oocytes. Scale bar, 20 μm. (D) Fluorescence intensity of γH2AX signals was recorded in young (n = 23), aged (n = 10), melatonin-administered (n = 16) and melatonin + luzindole-administered (n = 12) oocytes. (E) Representative images of apoptotic status shown by the Annexin-V staining in young, aged, melatonin-administered and melatonin + luzindole-administered oocytes. Scale bar, 20 μm. (F) The rate of early apoptosis was recorded in young (n = 65), aged (n = 42), melatonin-administered (n = 35) and melatonin + luzindole-administered (n = 39) oocytes. Control: oocytes from young mice; Vehicle: oocytes from aged mice administered with PBS; Mel: oocytes from aged mice administered with melatonin; Luz: oocytes from aged mice administered with melatonin and luzindole. Data were presented as mean percentage (mean ± SEM or SD) of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.