Abstract
Background
Substituting whole blood osmolality for plasma osmolality could expedite treatments otherwise delayed by the time required to separate erythrocytes from plasma. The purpose of this study was to compare the measured osmolality (mmol/kg) and calculated osmolarity (mmol/l) of whole blood and plasma.
Methods
The osmolality of whole blood and plasma was measured using freezing point depression by micro‐osmometer and osmolarity calculated from biosensor measures of sodium, glucose, and blood urea nitrogen. The influence of sample volume was also investigated post hoc by comparing measured osmolality at 20 and 250 μl.
Results
Sixty‐two volunteers provided 168 paired whole blood and plasma samples for analysis. The mean difference (whole blood − plasma; ±standard deviation) in osmolality was 10 ± 3 mmol/kg. Whole blood was greater than plasma in 168 of 168 cases (100%) and data distributions overlapped by 27%. The mean difference in osmolarity was 0 ± 2 mmol/l. Whole blood was greater than plasma in 90 of 168 cases (56%) and data distributions overlapped by 90%. The osmol gap (osmolality − osmolarity) was 16 ± 6 mmol for whole blood and 7 ± 5 mmol for plasma. Ten volunteers were tested on one occasion post hoc to investigate the potential effects of sample volume. The difference between whole blood and plasma was reduced to 3 ± 2 mmol/kg with a larger (250 μl vs. 20 μl) sample volume.
Conclusions
This investigation provides strong evidence that whole blood and plasma osmolality are not interchangeable measurements when a 20 μl sample is used.
Keywords: calculated osmolarity, measured osmolality, osmol gap, sample volume
INTRODUCTION
The measurement of plasma osmolality, whether alone or in conjunction with other body fluids and electrolytes, provides important diagnostic and prognostic value for a variety of disturbed states of body fluid balance 1, 2, 3, 4, 5, 6, 7, 8. The longstanding clinical significance of the plasma osmol gap 9, 10 is similarly appreciated 1, 11, 12 despite active debate concerning the most appropriate nomenclature 9, 13 and formulae 14, 15, 16, 17, 18, 19, 20. In emergency situations 21, 22 or those in which a field expedient test of osmolality is desirable 23, the use of whole blood osmolality could expedite treatment otherwise delayed by the time required to separate erythrocytes from plasma, particularly when very small sample volumes are required 21, 24.
The constancy of osmotic activity inside and outside living mammalian cells 25, 26, 27 strongly suggests that the osmolality of whole blood and plasma should be equivalent. Indeed, the molar concentration of individually dissolved substances per unit water volume (mmol/l) is the same between plasma and whole blood when molarity is determined by direct measures of substance activity or when other means are used so long as correction is made for differences in plasma and whole blood water fractions 7, 28, 29. The plasma molal concentration of all dissolved substances per unit water mass (osmolality, mmol/kg) will generally agree within 10 mmol units of the summed plasma molar concentrations of the primary osmotic substances (e.g., sodium, glucose, blood urea nitrogen) 10. This is true independent 15, 19 of whether a correction is applied for the difference in mass concentration of water 13. However, there is reason to speculate that osmometry might produce different results between whole blood and plasma. For example, whole blood has a significantly higher viscosity than plasma 30 and contains suspended particles that can act as nuclei for crystallization, thus potentially reducing freezing point and increasing osmolality 31. The more heterogeneous character of whole blood (vs. plasma) has long been suspected to be problematic where osmometry is concerned 32, and might be particularly challenging in situations where the sample volume is necessarily small 21, 24.
Despite the theoretical limitations of performing osmometry on whole blood samples, excellent agreement has been reported between whole blood and plasma in the form of small mean differences and large correlation coefficients 21, 22, 24, 32. A wide range of sample volumes has been used when considering both vapor pressure (5 μl) and freezing point depression (20–200 μl) techniques. The appearance of a close agreement may be somewhat misleading, however. In one study reporting approximate mean differences (whole blood–plasma) of less than 1–2 mmol/kg and a correlation coefficient of 0.96, individual differences were quite large (+22 to −18 mmol/kg) 22. In all studies in which a correlation coefficient was reported 21, 22, 24, 32, the relevant but wide range (70–200 mmol/kg) for osmolality measures may have also inflated the explained variance 33. A more recent study with dogs reported that whole blood osmolality was consistently 10 mmol/kg higher than plasma when a micro‐osmometer (20 μl) was used 34, which is similar to unpublished observations from our laboratory. In light of the limited details available for comparing agreement and the larger mean differences between whole blood and plasma reported more recently in dogs 34, it seems prudent to reconsider the general conclusion that whole blood and plasma are interchangeable quantities.
The purpose of this study was to compare plasma and whole blood on indices of measured osmolality and calculated osmolarity in order to better understand the potential for whole blood to replace plasma when there is a desire to rapidly measure the osmolality of small sample volumes. A secondary, post hoc, study purpose was to compare the results obtained using 20 and 250 μl sample volumes.
MATERIALS AND METHODS
Participants in this investigation were studied under ordinary living conditions (nearly free‐living) between 0800 and 1200 hr. Specific instructions were limited to requesting that they continue their ordinary food and fluid intake and physical activity patterns throughout their participation in the study. Study restrictions were limited to abstention from alcohol consumption for ≥24 h and food and fluid ≥90 min prior to each visit. The use of dietary supplements, and any medication other than an oral contraceptive was also prohibited. Volunteers were provided informational briefings and gave voluntary, informed written consent to participate. Investigators adhered to AR 70‐25 and US Army Medical Research and Materiel Command Regulation 70‐25 on the use of volunteers in research. The US Army Research Institute of Environmental Medicine Human Use Review Committee approved this study. Upon arrival to the laboratory, confirmation of adherence to study restrictions was obtained. Clothed body mass (kg) was then measured (stationary scale, model WSI‐600, Mettler Toledo, Toledo, OH), as well as standing height (cm; portable stadiometer, model 217, Seca, Hanover, MD) with shoes removed. Subjects were then seated and after a 20‐min stabilization period, blood was drawn from an antecubital vein without stasis.
Two blood samples were collected into 2.7 ml lithium‐heparin tubes (Sarstedt‐Monovette®, Newton, NC). One sample was set aside for immediate whole blood osmolality analysis, while the other sample was centrifuged at 1,500 × g for 15 min at 5°C to acquire plasma. A 20 μl aliquot sample of whole blood or plasma was immediately transferred to sample cuvettes for osmolality determination. Osmometry of whole blood and plasma was performed in triplicate via freezing‐point depression with a calibrated (ClinitrolTM 290 Reference Solution, Norwood, MA) micro‐osmometer (Fiske® Micro‐osmometer, Model 210, Norwood, MA) dedicated for blood analysis. When the triplicate intrasample measures differed by ≤3 mmol/kg (∼1%), the median value was used. If the triplicate intrasample measures differed by >3 mmol/kg, two additional samples were measured and the median value was used 35. Whole blood and plasma (150 μl) were also analyzed for sodium, potassium, calcium, magnesium, and blood urea nitrogen by direct ion‐selective electrode; glucose and lactate was analyzed by enzymatic determination; hematocrit was measured by conductivity with sodium correction and hemoglobin was measured by multiwavelength reflectance with conductivity correction; all analyses were performed using a Stat Profile Critical Care Xpress (Nova Biomedical, Waltham, MA). Plasma proteins were measured by refractometry (1110400A TS Meter, AO Reichert Scientific Instruments, Keene, NH). Osmolarity of whole blood and plasma was calculated from sodium, glucose, and blood urea nitrogen according to Mahon et al. 36. Plasma water concentration was calculated from plasma proteins 37 and whole blood water concentration from plasma water and hematocrit, assuming 71% water concentration for erythrocytes 29.
A secondary, post hoc research study question was investigated using a smaller number of volunteers in whom a single whole blood and plasma sample was obtained as described above. Samples were assayed only for osmolality, but were compared using both the 20 μl Fiske® Micro‐osmometer and a larger 250 μl Advanced® Model 3250 single sample osmometer (Norwood, MA). Data normality was tested using the D'Agostino‐Pearson omnibus test for skewness and kurtosis. Paired t‐tests were used to compare the statistical significance (P < 0.05) of mean differences between whole blood and plasma (osmolality and osmolarity) when investigating both the primary and secondary study questions. For the secondary study question, an appropriate Bonferroni correction was made (α/4 = 0.01) to account for the four comparisons made. The degree of distribution overlap was assessed to judge the importance of differences in the larger primary study sample only 38. Pearson correlation coefficients were used to examine simple associations. All parametric data are described using the mean ± standard deviation. Nonparametric data are represented using the median and (range).
RESULTS
Participants for this study included a heterogeneous sample of 62 healthy soldier and civilian volunteers (44 men, 18 women). Descriptive volunteer information included a median age of 24 years (19–46), height of 173 cm (117–193), and weight of 78.3 kg (48.8–111.8). All volunteers participated in one, two, or three test days. The median time interval between repeat days was 2 days (1–349). A total of 168 paired whole blood and plasma samples were obtained for analysis. A separate smaller group of 10 soldier and civilian volunteers (six men, four women) participated in the post hoc study investigation. Volunteers had a median age of 29 years (23–47), height of 175 cm (157–190), and weight of 73.9 kg (56.8–93.2).
Plasma proteins (75 ± 4 g/l), hematocrit (0.41 ± 0.03), and hemoglobin (141 ± 12 g/l) were within typical limits 39. Whole blood and plasma water concentrations were also typical at 84.0 ± 0.8% and 92.9 ± 0.3%, respectively. The analytical coefficient of variation for whole blood and plasma osmolality was calculated from ten triplicate measures [(standard deviation/mean) × 100] and was 0.6 and 0.5%, respectively. Table 1 provides a comparison of the molar concentrations of select substances in whole blood and plasma. Figure 1 provides a plot of whole blood (x‐axis) versus plasma (y‐axis) osmolality (A) and osmolarity (B). The mean difference (whole blood–plasma) in osmolality was 10 ± 3 mmol/kg (P < 0.001), whereby whole blood was always greater than plasma (168/168 or 100%). The mean difference in osmolarity was 0 ± 2 mmol/l (P < 0.05), whereby whole blood was greater than plasma in 90 of 168 cases (56%). The correlation coefficient between whole blood and plasma was the same (r = 0.75, P < 0.05) for osmolality and osmolarity. Figure 2A and B shows the Gaussian frequency distributions for whole blood and plasma osmolality and osmolarity. A compelling interpretation of the magnitude of the 10 mmol/kg difference between whole blood and plasma osmolality is that only 27% of the distributions overlap (73% uniqueness) 38. In contrast, 90% overlap exists for plasma and whole blood osmolarity (10% uniqueness). The osmol gap, calculated as measured osmolality minus calculated osmolarity, was 16 ± 6 mmol for whole blood and 7 ± 5 mmol for plasma.
Table 1.
Concentration (mmol/l) of Select Substances in Whole Blood and Plasma
| Whole blood | Plasma | |
|---|---|---|
| Sodium | 139 ± 1 | 139 ± 1 |
| Potassium | 4.2 ± 0.2 | 4.1 ± 0.3 |
| Chloride | 106 ± 2 | 107 ± 2 |
| Calciuma | 1.2 ± 0.0 | 1.2 ± 0.1 |
| Magnesiuma | 0.6 ± 0.0 | 0.6 ± 0.1 |
| Glucoseb | 5.5 ± 0.6 | 5.5 ± 0.6 |
| Lactate | 1.6 ± 0.7 | 1.5 ± 0.7 |
| BUN | 5.5 ± 1.4 | 5.7 ± 1.4 |
BUN, blood urea nitrogen.
Ionized.
Measured enzymatically; whole blood value multiplied by 1.11 to correct for differences in plasma and whole blood water concentrations (29).
Figure 1.
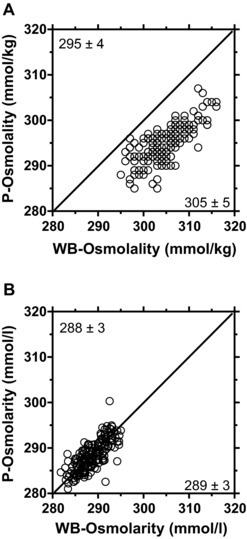
Relationship between whole blood (x‐axis) and plasma (y‐axis) osmolality (A) and osmolarity (B). The solid line represents the line of identity where y = x. The correlation coefficient was 0.75 for both panels (A) and (B). P, plasma; WB, whole blood. Values in the figure corners are the mean ± standard deviation for the x‐ and y‐axis data.
Figure 2.
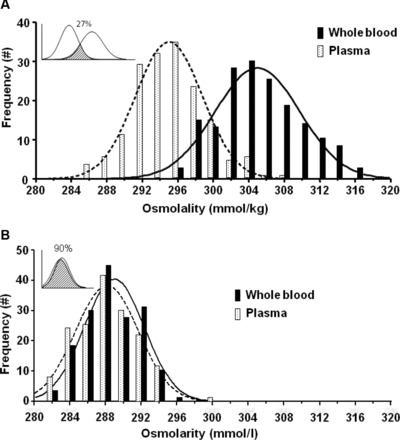
Gaussian frequency distributions for whole blood and plasma osmolality (A) and osmolarity (B). The shaded regions of the figure insets display shared overlap calculated according to reference (38).
Table 2 provides the results of the post hoc study investigation. The difference in whole blood osmolality between osmometers was 13 ± 4 mmol/kg (P < 0.01), while the difference in plasma osmolality between osmometers was 4 ± 2 mmol/kg (P < 0.01). The difference between whole blood and plasma osmolality was 12 ± 4 mmol/kg using the 20 μl micro‐osmometer (P < 0.01) and 3 ± 2 mmol/kg for the 250 μl osmometer (P < 0.01).
Table 2.
Effect of Sample Volume on Whole Blood and Plasma Osmolality Measurements (mmol/kg)
| 20 μl Sample volume | 250 μl Sample volume | |||
|---|---|---|---|---|
| Volunteer no. | Whole blood | Plasma | Whole blood | Plasma |
| 1 | 304 | 290 | 288 | 287 |
| 2 | 301 | 296 | 293 | 290 |
| 3 | 296 | 287 | 286 | 284 |
| 4 | 310 | 293 | 292 | 290 |
| 5 | 299 | 289 | 285 | 284 |
| 6 | 303 | 289 | 291 | 288 |
| 7 | 306 | 293 | 290 | 289 |
| 8 | 297 | 284 | 287 | 281 |
| 9 | 307 | 290 | 290 | 287 |
| 10 | 301 | 294 | 290 | 287 |
| Mean ± SD | 302 ± 5 | 291 ± 4 | 289 ± 3 | 287 ± 3 |
All means were statistically different from each other (P < 0.01), but differences ≤4 mmol/kg were considered marginal (40).
DISCUSSION
The primary purpose of this study was to compare whole blood and plasma on indices of measured osmolality and calculated osmolarity. In addition, we also investigated the potential impact of sample volume on measured osmolality in a post hoc study comparison. Our primary conclusion is that measured whole blood and plasma osmolality are significantly and meaningfully different when a 20 μl sample volume is used.
The molar concentration of individual substances was the same in whole blood and plasma (Table 1), consistent with the principle of osmotic constancy inside and outside living cells 25, 26, 27. The calculated osmolarity for plasma and whole blood, though significantly different due to the large sample size and paired design, also agreed within 1 mmol/l (Fig. 1B) and shared 90% distribution overlap (Fig. 2B). It is well known that the conversion of molarity to molality using summed quantities is complicated and imperfect, even when accounting for differences in the mass concentration of water 15, 19, 31. However, the mean osmol gap for plasma (7 mmol) was generally acceptable (<10 mmol) while the osmol gap for whole blood (16 mmol) was not 10. Barr et al. 34 employed the same formula 20 when calculating plasma and whole blood osmol gaps and found nearly identical results for plasma (8 mmol) and whole blood (18 mmol) as in this study. The generally accepted molal concentration equivalency of all dissolved substances in whole blood and plasma, when measured using osmometry 21, 22, 24, 32, was neither observed by Barr et al. 34 nor herein. Whole blood osmolality was consistently (168/168 observations), significantly (10 mmol/kg; P < 0.05), and meaningfully (only 27% distribution overlap) higher than plasma (Fig. 1A and Fig. 2A) and the whole blood osmol gap was unacceptably large (16 mmol).
At the completion of our primary study, we purchased a larger sample volume osmometer to follow‐up the possibility that a very small sample volume might explain our results. Table 2 provides strong additional support for our primary findings and also a definitive explanation. The difference between whole blood and plasma osmolality using a 20 μl sample volume in our group of 10 volunteers (12 ± 4 mmol/kg) was similar to our much larger group of 62 (10 ± 3 mmol/kg). However, the difference using a 250 μl sample volume, though significant, was as small (3 ± 2 mmol/kg) as the day‐to‐day variation in plasma osmolality 40 and smaller than plasma osmolality fluctuations associated with perturbed osmoregulation 40, 41, 42. The commonality of our results with those of Barr et al. 34 are in direct contrast to only one other study that used a 20 μl sample volume 24, the rest comprising either vapor pressure osmometry 21 or sample volumes between 100 and 200 μl 22, 32. The correlation coefficients calculated herein between whole blood and plasma osmolality were large (r = 0.75) and significant (P < 0.05), but comparatively smaller than those reported by Koumantakis and Weyland 24 (r = 0.91). The most likely explanation 33 lies in the smaller 27 mmol/kg range of plasma osmolality values in this study compared to the 70 mmol/kg range reported by Koumantakis and Weyland 24. Although larger plasma osmolality sample ranges have greater clinical relevance, our smaller range was still in excess of the typical reference interval for plasma osmolality 39. Other factors available for comparison (e.g., supplies or standard operating procedures) reveal no obvious explanation(s) for conflicting outcomes.
The more heterogeneous character of whole blood (vs. plasma) has long been suspected to be problematic where osmometry is concerned 32 and might be particularly challenging in situations where the sample volume is necessarily small 21, 24. In the present study, a clear difference was observed between measures of whole blood and plasma osmolality, but not osmolarity (Fig. 1A and B, and Fig. 2A and B), which we interpret as a genuine physical interference of whole blood during osmolality measurement using a 20 μl sample volume. Although Rocks et al. 32 clearly demonstrated that red blood cells themselves failed to impact measures of plasma osmolality (0–75% packed cells), this was demonstrated using 100 μl sample volumes. The large differences observed between whole blood and plasma osmolality using 20 μl, but not 250 μl samples, in this study support a volume‐dependent physical phenomenon.
CONCLUSION
In conclusion, this investigation provides strong evidence that whole blood and plasma osmolality are not interchangeable quantities when small (20 μl) sample volumes are compared using freezing point depression osmometry. Whole blood osmolality was consistently, significantly, and meaningfully higher than plasma osmolality and the osmol gap was unacceptably large. The magnitude of difference between whole blood and plasma osmolality observed in this study was reduced to marginal levels when using a larger (250 μl) sample volume. A physical interference of whole blood during osmolality measurement is suspected when a 20 μl sample volume is used. These findings have important implications for clinical laboratory testing when knowledge of blood osmolality is desired.
ACKNOWLEDGMENTS
The authors thank Elizabeth M. Caruso, Kurt J. Sollanek, Jeffery S. Staab, and Myra L. Jones for their expert assistance. The views, opinions, and/or findings contained in the article are those of the authors and should not be construed as an official Department of the Army position, or decision, unless so designated by other official documentation.
Grant sponsor: The United States Army Medical Research and Materiel Command (USAMRMC).
REFERENCES
- 1. Boyd D, Baker R. Osmometry: A new bedside laboratory aid for the management of surgical patients. Surg Clin of N Am 1971;51(1):241–250. [DOI] [PubMed] [Google Scholar]
- 2. Cheuvront SN, Kenefick RW, Sollanek KJ, Ely BR, Sawka MN. Water‐deficit equation: Systematic analysis and improvement. Am J Clin Nutr 2013;97(1):79–85. [DOI] [PubMed] [Google Scholar]
- 3. Davis JA, Harvey DR, Stevens JF. Osmolality as a measure of dehydration in the neonatal period. Arch Dis Child 1966;41(218):448–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med 1984;310(2):102–105. [DOI] [PubMed] [Google Scholar]
- 5. Haraway A, Becker E. Clinical application of cryoscopy. JAMA 1968;205(7):94–100. [PubMed] [Google Scholar]
- 6. Holtfreter B, Bandt C, Kuhn S, et al. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol Scand 2006;50(8):970–977. [DOI] [PubMed] [Google Scholar]
- 7. Langhoff E, Ladefoged J. Sodium activity, sodium concentration, and osmolality in plasma in acute and chronic renal failure. Clin Chem 1985;31(11):1811–1814. [PubMed] [Google Scholar]
- 8. Máttar J, Weil M, Shubin H, Stein L. Cardiac arrest in the critically ill. II. Hyperosmolal states following cardiac arrest. Am J Med 1974;56(2):162–168. [DOI] [PubMed] [Google Scholar]
- 9. Erstad B. Osmolality and osmolarity: Narrowing the terminology gap. Pharmacotherapy 2003;23(9):1085–1086. [DOI] [PubMed] [Google Scholar]
- 10. Kruse J, Cadnapaphornchai P. The serum osmole gap. J Crit Care 1994;9(3):185–197. [DOI] [PubMed] [Google Scholar]
- 11. McQuillen K, Anderson A. Osmol gaps in the pediatric population. Acad Emerg Med 1999;6(1):27–30. [DOI] [PubMed] [Google Scholar]
- 12. Rubin A, Braveman W, Dexter R, Vanamee P, Roberts K. The relationship between plasma osmolality and concentration in disease states. Clin Res Proc 1956;4:129. [Google Scholar]
- 13. Koga Y, Purssell R, Lynd L. The irrationality of the present use of the osmole gap. Toxicol Rev 2004;23(3):203–211. [DOI] [PubMed] [Google Scholar]
- 14. Bhagat C, Garcia‐Webb P, Fletcher E, Beilby J. Calculated vs measured plasma osmolalities revisited. Clin Chem 1984;30(10):1703–1705. [PubMed] [Google Scholar]
- 15. Dorwart WV, Chalmers L. Comparison of methods for calculating serum osmolality form chemical concentrations, and the prognostic value of such calculations. Clin Chem 1975;21(2):190–194. [PubMed] [Google Scholar]
- 16. Fazekas A, Funk G, Klobassa D, et al. Evaluation of 36 formulas for calculating plasma osmolality. Intensive Care Med 2013;39(2):302–308. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman R, Smilkstein M, Howland M, Goldfrank L. Osmol gaps revisited: Normal values and limitations. J Toxic: Clin Toxic 1993;31(1):81–94. [DOI] [PubMed] [Google Scholar]
- 18. Khajuria A, Krahn J. Osmolality revisited—Deriving and validating the best formula for calculated osmolality. Clin Biochem 2005;38(6):514–519. [DOI] [PubMed] [Google Scholar]
- 19. Rasouli M, Kalantari K. Comparison of methods for calculating serum osmolality: Multivariate linear regression analysis. Clin Chem Lab Med 2005;43(6):635–640. [DOI] [PubMed] [Google Scholar]
- 20. Worthley L, Guerin M, Pain R. For calculating osmolality, the simplest formula is the best. Anaesth Intens Care 1987;15(2):199–202. [DOI] [PubMed] [Google Scholar]
- 21. Assadi F. Validity of whole blood osmolality measurement in sick neonates. Pediatr Nephrol 1988;2(1):27–28. [DOI] [PubMed] [Google Scholar]
- 22. Weil H, Michaels S, Klein D. Meaurement of whole blood osmolality. Am J Clin Pathol 1982;77(4):447–448. [DOI] [PubMed] [Google Scholar]
- 23. Kratz A, Siegel A, Verbalis J, et al. Sodium status of collapsed marathon runners. Arch Pathol Lab Med 2005;129(2):227–230. [DOI] [PubMed] [Google Scholar]
- 24. Koumantakis G, Wyndham L. An evaluation of osmolality measurement by freezing point depression using micro‐amounts of sample. J Auto Chem 1989;11(2):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maffly R, Leaf A. The potential of water in mammalian tissues. J Gen Physiol 1959;42(6):1257–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Slyke D, Wu H, McLean F. Studies of gas and electrolyte equilibria in the blood. J Biol Chem 1923;56(3):765–849. [Google Scholar]
- 27. Yannet H, Darrow D, Cary M. The effect of changes in the concentration of plasma electrolytes on the concentration of electrolytes in the red blood cells of dogs, monkeys, and rabbits. J Biol Chem 1936;112(2):477–488. [Google Scholar]
- 28. Holtcamp H, Verhoef N, Leijnse B. The difference between the glucose concentrations in plasma and whole blood. Clinica Chimica Acta 1975;59(1):41–49. [DOI] [PubMed] [Google Scholar]
- 29. D'Orazio P, Burnett R, Fogh‐Andersen N, et al. Approved IFCC recommendation on reporting results for blood glucose. Clin Chem Lab Med 2006;44(12):1486–1490. [DOI] [PubMed] [Google Scholar]
- 30. Windberger U, Bartholovitsch A, Plasenzotti R, Korak K, Heinze G. Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: Reference values and comparison of data. Exp Physiol 2003;88(3):431–440. [DOI] [PubMed] [Google Scholar]
- 31. Sweeney T, Beuchat C. Limitations of methods of osmometry: Measuring the osmolality of biological fluids. Am J Physiol 1993;264(3 Pt. 2):R469–R480. [DOI] [PubMed] [Google Scholar]
- 32. Rocks B, Sherwood R, Cook J. Whole blood osmolality. Ann Clin Biochem 1986;23(Pt. 1):106–108. [DOI] [PubMed] [Google Scholar]
- 33. Westgard J, Hunt M. Use and interpretation of common statistical tests in method‐comparison studies. Clin Chem 1973;19(1):49–57. [PubMed] [Google Scholar]
- 34. Barr J, Pesillo‐Crosby S. Use of the advanced micro‐osmometer model 3300 for determination of a normal osmolality and evaluation of different formulas for calculated osmolarity and osmole gap in adult dogs. J Vet Emerg Crit Care 2008;18(3):270–276. [Google Scholar]
- 35. Bohnen N, Terwel D, Markerink M, Ten Haaf JA, Jolles J. Pitfalls in the measurement of plasma osmolality pertinent to research in vasopressin and water metabolism. Clin Chem 1992;38(11):2278–2280. [PubMed] [Google Scholar]
- 36. Mahon W, Holland J, Urowitz M. Hyperosmolar, non‐ketotic diabetic coma. Can Med Assoc J 1968;99(22):1090–1992. [PMC free article] [PubMed] [Google Scholar]
- 37. Eisenman A, Macheknzie L, Peters J. Protein and water of serum and cells of human blood, with a note on the measurement of red blood cell volume. J Biol Chem 1936;116(1):33–35. [Google Scholar]
- 38. Linacre J. Overlapping normal distributions. Rasch Measure Trans 1996;10(1):487–488. [Google Scholar]
- 39. Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 2004;351(15):1548–1563. [DOI] [PubMed] [Google Scholar]
- 40. Cheuvront SN, Ely BR, Kenefick RW, Sawka MN. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr 2010;92(3):565–573. [DOI] [PubMed] [Google Scholar]
- 41. Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 2008;9(7):519–531. [DOI] [PubMed] [Google Scholar]
- 42. Robertson GL, Aycinena P, Zerbe RL. Neurogenic disorders of osmoregulation. Am J Med 1982;72(2):339–353. [DOI] [PubMed] [Google Scholar]


