Figure 1.
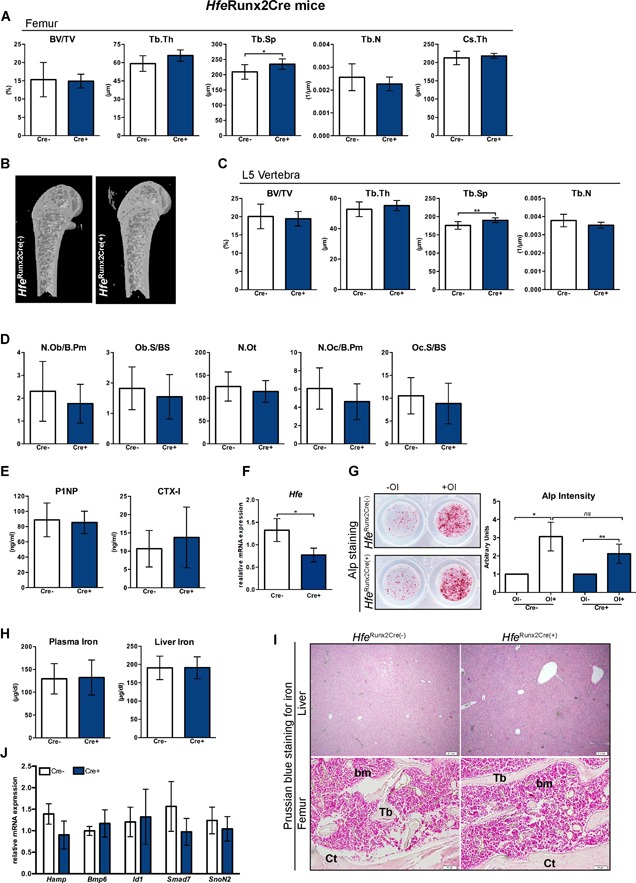
Hfe actions in osteoblasts are dispensable for the regulation of bone and iron metabolism. (A, C) µCT analysis of trabecular bone at distal femur and in the vertebra of Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 7; 10). (B) µCT 3D models of femora showing no evident changes in the composition of the trabecular bone, cortical bone, and marrow area. Images are representative of three mice per each group. (D) Histomorphometry showing no significant changes in osteoblast, osteocyte and osteoclast numbers between Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 6; 9). (E) The expression levels of bone formation marker P1NP, bone resorption marker C‐terminal collagen crosslinks telopeptide 1 (CTX‐I) in the serum of Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 6; 10). (F) Relative mRNA expression of Hfe in primary osteoblasts from Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 4; 5), measured by quantitative real‐time PCR (qRT‐PCR) and calculated relative to the expression of the reference gene Gapdh. (G) Alkaline phosphatase (ALP) staining and activity of primary osteoblasts from Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice cultured in the presence or absence of osteoblast differentiation medium (OI) for 7 days. ALP staining was performed at day 6. Representative images of primary osteoblasts derived from calvarias of neonatal Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 9; 9). ALP activity was measured in the supernatant at day 6 of osteoblast differentiation and was normalized to the cell viability. (H) Circulating iron levels and the nonheme liver iron content in Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 7; 10). (I) Prussian blue staining for iron depositions in the liver and femoral bone (decalcified) of Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice. Images are representative staining of three mice per each group. The pictures represent 40× (liver) and 100× (bone) magnification. (J) Relative mRNA expression of iron genes in the liver of Hfe Runx2Cre(+) and Hfe Runx2Cre(−) mice (n = 4; 5), measured by qRT‐PCR and calculated relative to the expression of the reference gene Gapdh. Data were analyzed using GraphPad Prism software and results are shown as mean ± SD. For the statistical analysis, a nonparametric distribution and the Mann‐Whitney U test were used. *p values < .05, **p values < .01. All mice were males 13 weeks of age. BV/TV = bone volume/tissue volume; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; Tb.N = trabecular number; Tb = trabecular bone; Ct = cortical bone; bm = bone marrow; N.Ob/B.Pm = osteoblast number/bone perimeter; Ob.S/BS = osteoblast surface/bone surface; N.Ot = osteocyte number; N.Oc/B.Pm = osteoclast number/bone perimeter; Oc.S/BS = osteoclast surface/bone surface; OI = osteogenic induction.
