Figure 1. MTAP loss in transformed astrocytes promotes tumorigenic, CD133-positive cells.
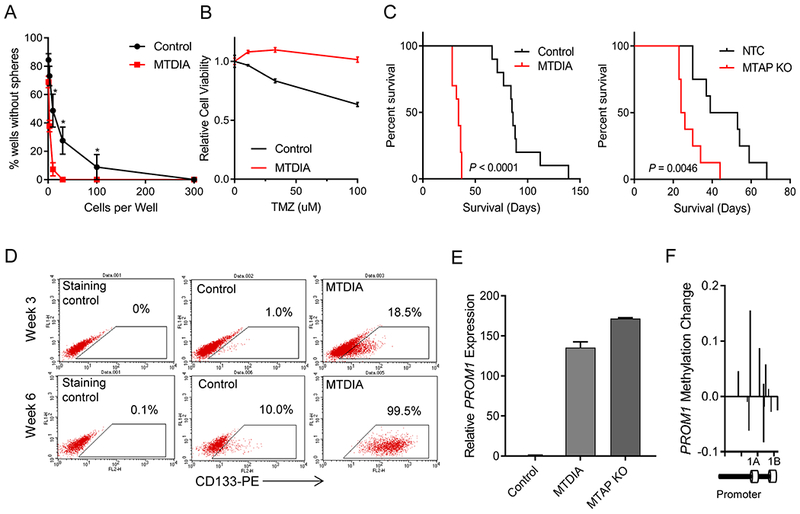
(A) Long-term MTAP inhibition with MTDIA results in cells with greater sphere-forming capacity. Average of 3 experiments is shown and error bars denote SEM. Stem cell frequency calculated by Extreme Limiting Dilution Analysis (ELDA) shows stem cell frequency in MTDIA treated cells of 1/3.82 cells (95% Confidence Interval 1/2.94-1/5.03) and in control cells is 1/21.95 (95% Confidence Interval 1/16.34-1/29.55), chisq = 78.3, P = 8.8×10−19 (B) Transformed astrocytes that had been transformed with or without MTDIA in the culture media were treated with varying doses of temozolomide for 3 days and cell viability was measured by CCK8 (n=6, ANOVA P value < 1×10−4). (C) Left: Kaplan-Meier survival curves show increased tumorigenicity of astrocytes treated with MTDIA during the process of transformation with OMRP (transformation #1) in nude mice (log-rank P value < 1×10−4). Right: CRISPR-mediated genetic knockout of MTAP in astrocytes during transformation (transformation #6), also results in increased tumorigenic potential (log-rank P value = 0.0046). (D) Flow cytometry measurement of CD133 expression shows progressive increase following astrocyte transformation that is enhanced by MTAP inhibition with MTDIA. (E) MTAP knockout or treatment with MTAP inhibitor MTDIA resulted in increased PROM1 transcription measured by RT-PCR at 6 weeks after transformation (F) Methylation of PROM1 promoter in parental transformed astrocytes relative to MTDIA treated astrocytes measured by the MethylationEPIC array. Control astrocytes maintain higher PROM1 methylation levels than MTDIA treated cells.
