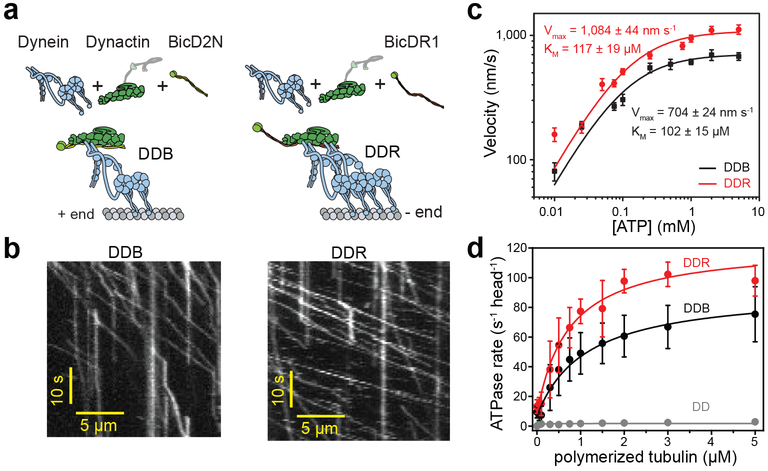
Figure 1. DDR hydrolyzes ATP and moves faster than DDB.
(a) Schematic depiction of the DDB and DDR complexes. The N-terminal coiled-coil domain of BicD2N primarily recruits single dynein, while BicDR1 recruits two dyneins to dynactin. (b) Sample kymographs show the motility of DDB and DDR complexes on MTs (nDDB = 209, nDDR = 223, three independent experiments per condition). (c) Average velocities of DDB and DDR as a function of ATP concentration (mean ± s.e.m., n = 72, 84, 74, 78, 84, 82, 98, 80, 70 motors for DDB; and 70, 75, 72, 78, 106, 86, 100, 92, 98, 80 motors for DDR from left to right, three independent experiments per condition). Data fit well to Michaelis-Menten kinetics with a Hill coefficient of 1 (solid curves, ± s.e. of the fit). (d) MT-stimulated ATPase rates of dynein-dynactin (DD), DDB, and DDR (mean ± s.d.) fit well to Michaelis-Menten kinetics with a Hill coefficient of 1 (solid curves, three independent experiments).