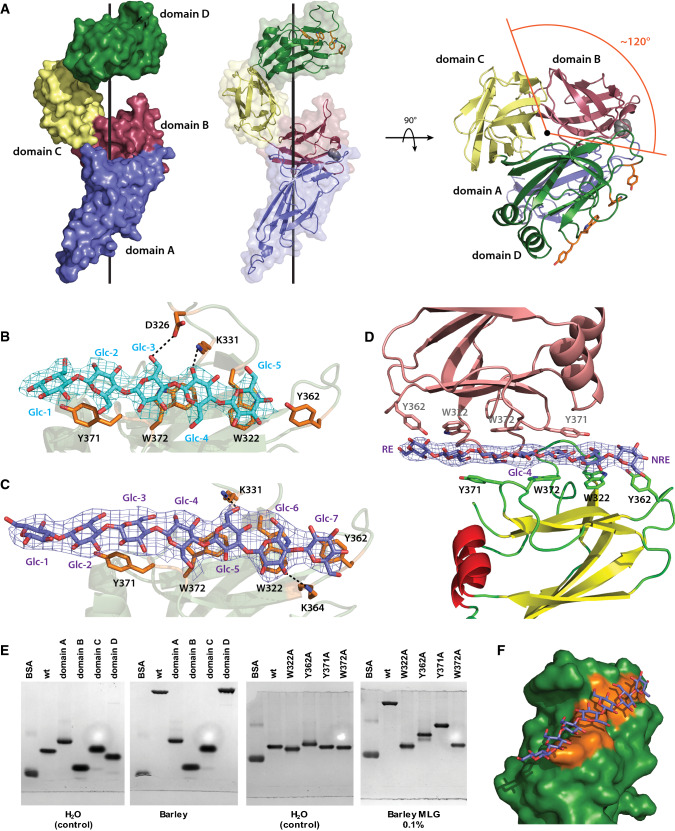
Fig. 4.
BoSGBPMLG-B crystal structure. a Overall structure of BoSGBPMLG-B in surface/cartoon representation with each domain colored differently: domain A—blue, domain B—raspberry, domain C—pale yellow, domain D—forest. A side view and a top view are shown with the black line representing the imaginary axis around which the domains wrap. The single interdomain proline is shown as gray spheres and the aromatic sidechains comprising the binding platform are shown as orange sticks. b Close-up of the binding site of the cellohexaose complex with interacting residues shown as opaque orange sticks. Cellohexaose is colored cyan and potential hydrogen bonding interactions are shown as black dashed lines (within 3.5 Å of the ligand). Omit map for the ligand (generated by Privateer [79]) is shown contoured to 3σ. Of the four molecules in the asymmetric unit, data from chain A is shown a representative. c Close-up of the binding site of the MLG7 complex with interacting residues shown as opaque orange sticks. MLG7 is colored slate and potential hydrogen bonding interactions are shown as black dashed lines (within 3.5 Å of the ligand). Omit map for the ligand is shown contoured to 3σ. Of the four molecules in the asymmetric unit, data from chain D are shown as representative. d A single MLG7 ligand being shared between two BoSGBPMLG-B molecules belonging to neighboring asymmetric units. The bottom molecule is colored according to secondary structure (yellow β-strands, red α-helices, and green loops), the top molecule from a different asymmetric unit is colored salmon, and the ligand is colored slate. Omit map for the ligand is shown contoured to 3σ. RE reducing end, NRE non-reducing end. An analogous orientation was observed for the cellohexaose complex (not shown, PDB ID 6E57). e Affinity gel electrophoresis of individual BoSGBPMLG-B domains and binding platform site-directed mutants. f Surface representation of the binding platform of domain D in complex with MLG7. Aromatic sidechains comprising the binding platform are colored orange and MLG7 is colored slate