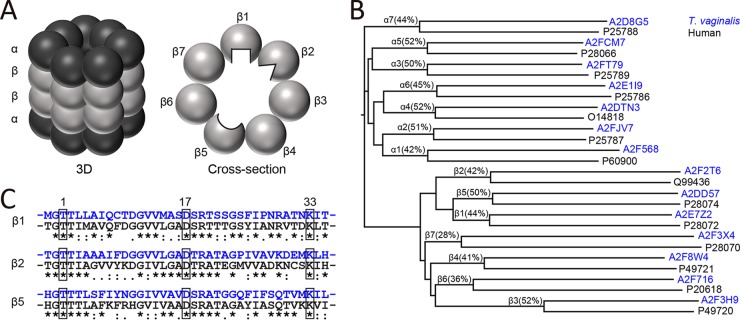
FIG 3.
Bioinformatic analysis of T. vaginalis proteasome subunits. (A) Scheme of the mammalian 20S proteasome in three dimensions (left) and as a planar cross-section of a β ring containing the three enzymatically active subunits (right). The β1, β2, and β5 active sites face the inner core of the proteasome barrel. Each subunit has a unique substrate and inhibitor-binding preference, as indicated by differently shaped active sites. (B) Alignment dendrogram of putative T. vaginalis proteasome subunits (UniProt accession numbers in blue) and α and β subunits of the human proteasome (UniProt accession numbers in black). The percentage of sequence identity for each pair is noted. (C) Sequence alignment of the N-terminal amino acids of the mature human β1, β2, and β5 subunits (black) with the homologous T. vaginalis proteins (blue). The active-site residues in positions 1 (Thr), 17 (Asp), and 33 (Lys) are boxed.