Abstract
Rationale: Body composition changes throughout life may explain the inconsistent associations reported between body mass index and lung function in children.
Objectives: To assess the associations of body weight and composition trajectories from 7 to 15 years with lung function at 15 years and lung function growth between 8 and 15 years.
Methods: Sex-specific body mass index, lean body mass index, and fat mass index trajectories were developed using Group-Based Trajectory Modeling on data collected at least twice between 7 and 15 years from 6,964 children (49% boys) in the UK Avon Longitudinal Study of Parents and Children birth cohort. Associations of these trajectories with post-bronchodilation lung function parameters at 15 years and with lung function growth rates from 8 to 15 years were assessed using multivariable linear regression models, stratified by sex, in a subgroup with lung function data (n = 3,575).
Measurements and Main Results: For all body mass measures we identified parallel trajectories that increased with age. There was no consistent evidence of an association between the body mass index trajectories and lung function measures. Higher lean body mass index trajectories were associated with higher levels and growth rates of FVC, FEV1, and forced expiratory flow, midexpiratory phase in both sexes (e.g., boys in the highest lean body mass index trajectory had on average a 0.62 L [95% confidence interval, 0.44–0.79; P trend < 0.0001] higher FVC at 15 yr than boys in the lowest trajectory). Increasing fat mass index trajectories were associated with lower levels and growth rates of FEV1 and forced expiratory flow, midexpiratory phase only in boys and lower levels of FEV1/FVC in both sexes.
Conclusions: Higher lean body mass during childhood and adolescence is consistently associated with higher lung function at 15 years in both sexes, whereas higher fat mass is associated with lower levels of only some lung function parameters.
Keywords: ALSPAC, children, epidemiology, respiratory health
At a Glance Commentary
Scientific Knowledge on the Subject
Previous studies have shown inconsistent results regarding the association of overweight/obesity with lung function in children and adolescents, likely because most have defined overweight/obesity using the body mass index. However, the body mass index does not distinguish between different components of body weight (e.g., fat mass and lean body mass). The few studies that have assessed the role of body composition on lung function in children and adolescents are all cross-sectional and based on specific populations (subjects with asthma, obese children, cystic fibrosis), and most did not consider the role of relevant confounders, such as previous lung function levels, pubertal status, physical activity, and diet.
What This Study Adds to the Field
This longitudinal study uses data from a large population-based birth cohort with repeated objective measures of body composition and information on numerous relevant confounders to show that higher lean body mass during childhood and adolescence is associated with higher levels of FEV1, FVC, and forced expiratory flow, midexpiratory phase at 15 years and with higher growth rates of these parameters from 8 to 15 years. Higher fat mass was associated with lower levels and growth rates of FEV1 and forced expiratory flow, midexpiratory phase only in boys and lower levels of FEV1/FVC in both sexes. Our study highlights the importance of assessing body composition, and not just body mass index, when studying the respiratory health effects of body weight in children and adolescents.
Lung function is a powerful marker of overall health and a significant predictor of future morbidity and mortality in the general population (1). Because lung function levels in childhood predict adult lung function, identifying factors that influence the development of lung function in childhood is important. Given the current global increase of childhood overweight and obesity, several studies have assessed their associations with lung function, but findings are inconsistent. Some studies report a positive association of overweight and obesity, as measured by body mass index (BMI), with lung function, whereas others show a negative association (2–8). An important limitation of these studies is that they did not distinguish between lean body mass and fat mass, both of which contribute to the composite measure BMI.
Some studies have examined body composition measures in relation to lung function, but they were all cross-sectional, most focused on specific populations (cystic fibrosis, obese children, or children with asthma) and most did not consider pubertal status, physical activity, or diet as relevant confounders (9–16). Furthermore, they only considered measurements at a single time point, and did not capture changes in the proportion of the different components of body weight (e.g., fat mass, lean body mass, bone mass) that occur over time and vary with sex (17).
Here we assess the association of body weight and composition trajectories, defined using repeated anthropometric and dual-energy X-ray absorptiometry scanner measures taken from age 7 to 15 years, with lung function at 15 years and lung function growth between 8 and 15 years, using data from the UK population-based Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort. This approach overcomes the limitations of previous research.
Some of the results of this study have been previously reported in the form of an abstract to the European Respiratory Society annual congress (18).
Methods
Complete details are provided in the online supplement.
Study Population
We used data from the 14,305 singleton births recruited in the population-based UK ALSPAC birth cohort, previously described (19, 20). For the identification of body weight and composition trajectories, we included children with at least two repeated measures of body weight and composition between the ages of 7 and 15 years (n = 6,964). Children who additionally had lung function measures at age 15 years were used to evaluate associations of body weight and composition trajectories with lung function measures at 15 years (n = 3,575) (see Figure E1 in the online supplement).
The ALSPAC Ethics and Law Committee and the Local Research Ethics Committees gave ethical approval. All participants and their parents/guardians provided written informed consent.
Measures
Body weight, height, and composition were assessed following standardized procedures (21). Weight and height were measured every year from age 7 to 15 years. Body composition (total lean body mass, total fat mass, and total bone mass) was measured using a Lunar Prodigy dual-energy X-ray absorptiometry scanner at age 9, 11, 13, and 15 years. BMI, lean body mass index (LBMI), and fat mass index (FMI) were calculated by dividing body weight, total lean body mass, and total fat mass (kg) by height (m) squared, respectively.
Lung function was measured by spirometry at 8 and 15 years (Vitalograph 2120; Vitalograph) according to American Thoracic Society standards (22). At 15 years, lung function was measured before and after bronchodilation with salbutamol. FVC, FEV1, and forced expiratory flow, midexpiratory phase (FEF25–75) were obtained and the FEV1/FVC ratio was calculated. The outcomes of the analysis were: post-bronchodilation lung function measures at 15 years and rate of lung function growth from age 8 to 15 years (calculated as [prebronchodilation lung function at 15 yr – prebronchodilation lung function at 8 yr]/time of follow-up in yr).
We collected information, at different time points, on maternal social class, birthweight, gestational age, breastfeeding, tobacco exposure (during pregnancy, childhood, and first hand), total dietary energy intake, physical activity (by accelerometer), asthma doctor-diagnosis, and pubertal status.
Statistical Analysis
We conducted all analyses stratified by sex as body weight and composition as well as lung function have been found to differ across sexes.
We identified BMI trajectories by applying a Group-Based Trajectory Modeling approach (23) using yearly data from ages 7 to 15 years, and LBMI and FMI trajectories using data from ages 9, 11, 13, and 15 years. Because the distribution of BMI and FMI was right-skewed, we applied the natural log-transformation to all body weight and composition measures before the identification of the trajectories. The assigned trajectory was used as the exposure variable in all subsequent analyses.
Associations of body weight and composition trajectories with post-bronchodilation lung function measures at age 15 years and lung function growth rates from age 8 to 15 years were examined using multivariable linear regression. The final multivariable models included adjustments for maternal social class, maternal smoking during pregnancy, birth weight, any breastfeeding, pubertal status, and age and height at 15 years. We additionally adjusted all models for lung function levels at 8 years to reduce potential reverse causality. The models for the LBMI and FMI trajectories were also mutually adjusted.
We conducted several sensitivity analyses to assess the sensitivity of our estimates to varying assumptions regarding selection bias, information bias, and confounding (see online supplement).
All analyses were conducted using Stata/SE version 12.0 (StataCorp).
Results
Characteristics of Study Sample
We included 6,964 children (49.0% boys) in the identification of the body weight and composition trajectories. These children were more likely to be girls, have a higher socioeconomic status, a higher birth weight, a higher proportion of breastfeeding, and lower maternal smoking exposure than the children not included in the present analysis but participating in ALSPAC. Additionally, boys had lower LBMI and girls had lower BMI at 9 years when compared with the children not included in our analysis (see Table E1). A subset of the included children with available spirometry was used to analyze the associations of body weight and composition trajectories with lung function at 15 years (n = 3,575; 47.2% boys). The children in this subgroup were more likely to be girls and have a higher socioeconomic status, a higher proportion of breastfeeding, and lower maternal smoking exposure than those not included, but they did not differ in terms of body weight and composition trajectories or in baseline lung function measures (see Table E2).
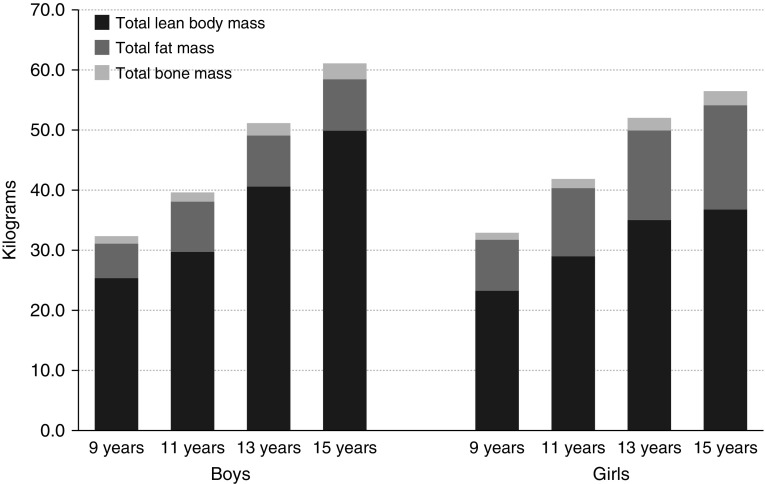
Table 1 shows the main characteristics of the sample subset used in the association analysis with lung function. Approximately half of the mothers had a high social class and around 16% smoked during pregnancy. Boys had significantly higher lung function levels (FVC, FEV1, and FEF25–75) at 8 and 15 years and higher lung function growth between 8 and 15 years than girls. Figure 1 and Table 2 show the body weight and composition characteristics of the children across ages. Body weight was composed mainly of lean body mass at all ages for both boys and girls. The amount of lean body mass and fat mass changed over time, although this pattern differed by sex. Boys had lower BMI and FMI, but higher LBMI, than girls at all ages.
Table 1.
Characteristics of the Participants Used to Assess Associations of Body Weight and Composition Trajectories with Lung Function at 15 Years
| Total (n = 3,575) | Boys (n = 1,687) | Girls (n = 1,888) | P Value | |
|---|---|---|---|---|
| Potential confounders | ||||
| Maternal social class | 2,941 | |||
| Professional and intermediate | 1,322 | 639 (46.2) | 683 (43.8) | 0.712 |
| Skilled nonmanual | 1,179 | 554 (40.1) | 625 (40.1) | |
| Skilled manual, partly skilled, and unskilled | 440 | 190 (13.7) | 250 (16.1) | |
| Maternal smoking during pregnancy | 3,278 | 260 (16.8) | 290 (16.7) | 0.930 |
| Birth weight, g | 3,381 | 3,485 (3,160 to 3,860) | 3,402 (3,120 to 3,700) | <0.0001 |
| Birth weight, z-score* | 3,366 | 0.5 (1.1) | 0.5 (1.0) | 0.686 |
| Gestation, wk | 3,425 | 40 (39 to 41) | 40 (39 to 41) | <0.0001 |
| Ever breastfed | 3,335 | 1,385 (88.1) | 1,521 (86.3) | 0.137 |
| Total energy intake at 7 yr, kcal | 3,004 | 1,758 (1,586 to 1,973) | 1,630 (1,457 to 1,819) | <0.0001 |
| Wear-time in MVPA at 11 yr, min | 2,955 | 24.4 (15.4 to 36.5) | 15.6 (9.4 to 24.7) | <0.0001 |
| Smoking at 14 yr | 2,790 | 42 (3.4) | 109 (7.0) | <0.0001 |
| Age at 15 yr, yr | 3,575 | 15.3 (15.3 to 15.5) | 15.3 (15.3 to 15.5) | 0.015 |
| Height at 15 yr, cm | 3,538 | 174.4 (169.4 to 179.2) | 164.4 (160.6 to 168.6) | <0.0001 |
| Height at 15 yr, z-score† | 3,538 | 0.4 (1.0) | 0.4 (0.9) | 0.017 |
| Lifetime doctor-diagnosed asthma | 3,573 | 443 (26.3) | 422 (22.4) | 0.006 |
| Pubertal status | ||||
| Age at menarche, yr | 1,701 | — | 12.7 (11.8 to 13.6) | — |
| Voice break status at age 15 yr | 1,649 | |||
| Not yet started | 218 | 218 (13.2) | — | — |
| Starting to break | 505 | 505 (30.6) | — | — |
| Completely broken | 926 | 926 (56.2) | — | — |
| Lung function measures (raw data) | ||||
| 8 yr (prebronchodilation) | ||||
| FVC, L | 3,078 | 2.0 (0.3) | 1.8 (0.3) | <0.0001 |
| FEV1, L | 3,045 | 1.7 (0.3) | 1.6 (0.3) | <0.0001 |
| FEF25–75, L/s | 3,078 | 2.0 (0.5) | 2.1 (0.5) | 0.017 |
| FEV1/FVC, % | 3,045 | 87.3 (6.8) | 89.4 (6.0) | <0.0001 |
| 15 yr (post-bronchodilation) | ||||
| FVC, L | 3,567 | 4.2 (0.9) | 3.3 (0.6) | <0.0001 |
| FEV1, L | 3,433 | 3.8 (0.8) | 3.1 (0.6) | <0.0001 |
| FEF25–75, L/s | 3,575 | 4.7 (1.2) | 4.0 (1.0) | <0.0001 |
| FEV1/FVC, % | 3,433 | 91.1 (6.6) | 93.0 (6.3) | <0.0001 |
| Lung function measures, z-scores‡ | ||||
| 8 yr (prebronchodilation) | ||||
| FVC, L | 2,807 | −0.04 (1.1) | −0.03 (1.0) | 0.853 |
| FEV1, L | 2,775 | −0.03 (1.0) | 0.02 (1.0) | 0.173 |
| FEF25–75, L/s | 2,807 | −0.11 (1.1) | −0.13 (1.0) | 0.736 |
| FEV1/FVC, % | 2,775 | 0.03 (1.1) | 0.07 (1.0) | 0.322 |
| 15 yr (post-bronchodilation) | ||||
| FVC, L | 3,245 | −0.87 (1.3) | −0.97 (1.3) | 0.024 |
| FEV1, L | 3,123 | −0.34 (1.3) | −0.58 (1.3) | <0.0001 |
| FEF25–75, L/s | 3,253 | 0.16 (1.1) | 0.08 (1.2) | 0.033 |
| FEV1/FVC, % | 3,123 | 0.91 (1.1) | 0.76 (1.1) | 0.0002 |
| Prebronchodilation lung function growth rates from age 8 to 15 yr§ | ||||
| FVC, ml/yr | 3,073 | 325.5 (105.8) | 214.4 (72.7) | <0.0001 |
| FEV1, ml/yr | 3,013 | 293.8 (94.3) | 200.7 (66.4) | <0.0001 |
| FEF25–75, (ml/s) · yr | 3,070 | 327.4 (139.8) | 234.2 (115.4) | <0.0001 |
Definition of abbreviations: FEF25–75 = forced expiratory flow, midexpiratory phase; MVPA = moderate to vigorous physical activity.
Data are shown as mean (SD), median (25th–75th percentiles), or n (%). Em dashes indicate “not relevant.”
P values were determined by the chi-square test, Student’s t test, or Mann-Whitney test comparing distributions across sexes. Bold values indicate P < 0.05.
Derived using the International Fetal and New-born Growth Consortium for the 21st Century standards. Note that 15 children had missing values for birth weight z-score because they did not have information for gestational age, which should be included in the equation.
Derived using the World Health Organization Child Growth Standards.
Derived using the Global Lung Initiative equations.
Rate of lung function growth for each parameter was calculated as: (prebronchodilation lung function measure at 15 yr − prebronchodilation lung function measure at 8 yr)/time of follow-up in years.
Figure 1.
Distribution of body weight components from age 9 to 15 years, stratified by sex. Body weight components were measured using a dual-energy X-ray absorptiometry scanner. The presented values are the median of total lean body mass, total fat mass, and total bone mass.
Table 2.
Descriptive Statistics of Body Weight and Composition Measures of the Participants Used to Assess Associations of Body Weight and Composition Trajectories with Lung Function at 15 Years
| n (N = 3,575) | Boys (n = 1,687) [Median (P25–P75)] | Girls (n = 1,888) [Median (P25–P75)] | P Value | |
|---|---|---|---|---|
| Body weight measures | ||||
| BMI, kg/m2 | ||||
| 7 yr | 3,261 | 15.8 (14.9–16.8) | 15.9 (14.9–17.3) | 0.007 |
| 8 yr | 2,981 | 16.5 (15.5–17.9) | 16.8 (15.5–18.5) | 0.007 |
| 9 yr | 3,323 | 16.8 (15.6–18.6) | 17.3 (15.8–19.2) | <0.0001 |
| 10 yr | 3,363 | 17.3 (15.9–19.3) | 17.7 (16.1–19.9) | 0.002 |
| 11 yr | 3,389 | 18.0 (16.5–20.4) | 18.6 (16.8–21.0) | <0.0001 |
| 12 yr | 3,325 | 18.7 (17.1–21.0) | 19.4 (17.6–21.8) | <0.0001 |
| 13 yr | 3,326 | 19.3 (17.7–21.5) | 20.1 (18.4–22.4) | <0.0001 |
| 15 yr | 3,533 | 20.4 (18.8–22.5) | 21.1 (19.4–23.4) | <0.0001 |
| Body composition measures | ||||
| LBMI, kg/m2 | ||||
| 9 yr | 3,197 | 13.0 (12.4–13.6) | 12.1 (11.5–12.7) | <0.0001 |
| 11 yr | 3,361 | 13.3 (12.6–14.0) | 12.7 (11.9–13.5) | <0.0001 |
| 13 yr | 3,293 | 14.9 (13.9–16.0) | 13.4 (12.7–14.2) | <0.0001 |
| 15 yr | 3,516 | 16.3 (15.3–17.3) | 13.6 (12.8–14.3) | <0.0001 |
| FMI, kg/m2 | ||||
| 9 yr | 3,197 | 3.0 (2.1–4.6) | 4.3 (3.1–6.1) | <0.0001 |
| 11 yr | 3,361 | 3.7 (2.5–5.9) | 4.9 (3.5–7.0) | <0.0001 |
| 13 yr | 3,293 | 3.1 (2.1–5.2) | 5.7 (4.2–7.6) | <0.0001 |
| 15 yr | 3,516 | 2.8 (1.9–4.6) | 6.5 (5.0–8.4) | <0.0001 |
Definition of abbreviations: BMI = body mass index; FMI = fat mass index; LBMI = lean body mass index; P25–P75 = 25th–75th percentiles.
P values were determined by the Mann-Whitney test comparing distributions across sexes. Bold values indicate P < 0.05.
Body Weight and Composition Trajectories
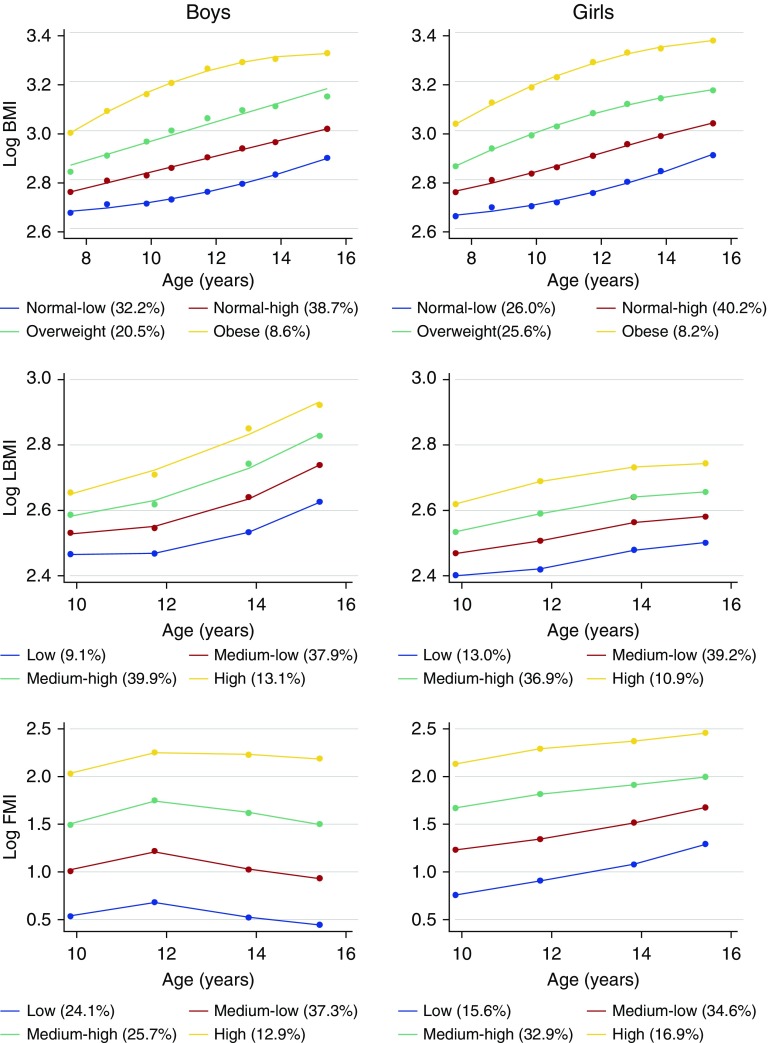
In both boys and girls, we identified four parallel BMI trajectories from 7 to 15 years. For both sexes, BMI increased with age (Figure 2; see Table E3). According to the World Health Organization reference cutoffs (24), we labeled these trajectories as “normal-low,” “normal-high,” “overweight,” and “obese.” In boys, the median BMI increased from 14.6 kg/m2 at age 7 years to 18.3 kg/m2 at age 15 years in the “normal-low” BMI trajectory and from 20.1 kg/m2 at age 7 years to 27.7 kg/m2 at age 15 years in the “obese” BMI trajectory (see Table E3).
Figure 2.
Sex-specific body weight and composition trajectories from 7 to 15 years. The percentage of the sample that is included in each trajectory is reported in the legend. The y-axis represents the natural log-transformed levels of body mass index (BMI), lean body mass index, and fat mass index (the equivalent raw values can be calculated by exponentiation of the log-transformed values [i.e., BMI raw value = exp(log BMI)]). FMI = fat mass index; LBMI = lean body mass index.
For LBMI, we identified four parallel trajectories from age 9 to 15 in both sexes (Figure 2; see Table E4). According to reference curves for body composition in children (25), we labeled these trajectories as “low,” “medium-low,” “medium-high,” and “high.” Median LBMIs were consistently greater in boys than girls for all trajectories. Also, the increase per year of LBMI was steeper in boys than girls, specifically between age 11 and 15 years.
For FMI, we identified four parallel trajectories from age 9 to 15 in both sexes, which we labeled “low,” “medium-low,” “medium-high,” and “high” (Figure 2; see Table E5) (25). Median FMIs were consistently greater in girls than boys for all trajectories. In boys, FMI levels consistently increased from age 9 to 11 years and then slightly declined from age 11 years onward. In girls, FMI consistently increased up to 15 years in all trajectories.
Associations of Body Weight and Composition Trajectories with Post-bronchodilation Lung Function at 15 Years
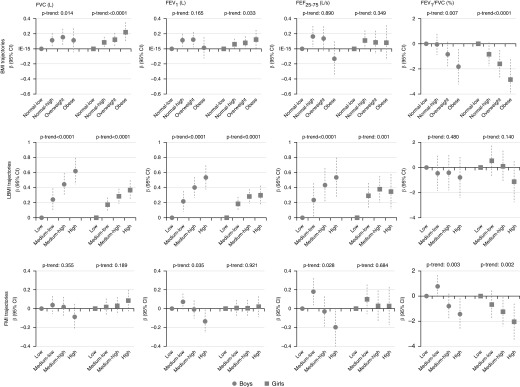
Adjusted associations between the BMI trajectories and post-bronchodilation lung function measures at age 15 years were inconsistent. Significant associations were only apparent for some trajectories and some lung function parameters (Figure 3; see Table E6).
Figure 3.
Sex-specific associations of body weight and composition trajectories with post-bronchodilation lung function measures at age 15 years. Models are adjusted for maternal social class, maternal smoking during pregnancy, birth weight, breastfeeding, lung function measures at 8 years, pubertal status (age at menarche for girls and voice break status at age 15 yr for boys), and age and height at 15 years. Models for fat mass index and lean body mass index are also mutually adjusted. β = estimate of regression coefficient; BMI = body mass index; CI = confidence interval; FEF25–75 = forced expiratory flow, midexpiratory phase; FMI = fat mass index; LBMI = lean body mass index.
Both boys and girls in the highest LBMI trajectories had higher FVC, FEV1, and FEF25–75. The association between the LBMI trajectories and these lung function variables exhibited a linear dose–response pattern (e.g., boys in the “medium-low,” “medium-high,” and “high” LBMI trajectory groups had on average a 0.24 L 95% confidence interval [0.09–0.39], 0.44 L [0.29–0.59], and a 0.62 L [0.44–0.79] higher FVC respectively than boys in the “low” LBMI trajectory [P-trend < 0.0001]). We did not find any statistically significant association between the LBMI trajectories and the FEV1/FVC ratio for either sex (Figure 3; see Table E6).
Boys in the “high” FMI trajectory had lower FEV1 (−0.14 L [−0.26 to −0.01]; P = 0.032) than boys in the “low” FMI trajectory and there was a trend toward lower FEF25–75 with higher FMI trajectories (P-trend = 0.028). We did not find any statistically significant association between FMI trajectories and FEV1 or FEF25–75 in girls, nor between FMI trajectories and FVC in boys or girls. Both boys and girls who were in the highest FMI trajectory exhibited lower FEV1/FVC ratios (Figure 3; see Table E6).
All sensitivity analyses showed very similar results for LBMI (see Tables E8–E13), even after additional adjustment for physical activity and total energy intake (see Table E8). For the FMI trajectories, the association between a higher FMI trajectory and a lower FEV1/FVC ratio was maintained in all sensitivity analysis, but the associations with the other lung function parameters were more instable: first, the magnitude of the associations of FMI with FEV1 and FEF25–75 (observed only in boys in the main analysis) was attenuated in some of the analyses and second, an association appeared between the “high” FMI trajectory and post-bronchodilation FVC in girls only in some of the models.
Associations of Body Weight and Composition Trajectories with Prebronchodilation Lung Function Growth Rates from Age 8 to 15 Years
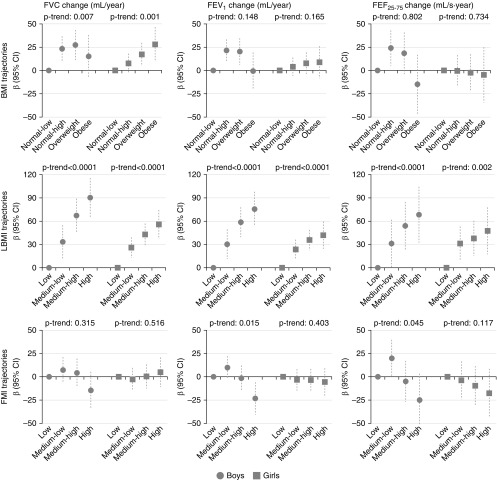
After adjusting for relevant confounders, there was no evidence of a consistent association between BMI trajectories and lung function growth rate (Figure 4; see Table E7).
Figure 4.
Sex-specific associations of body weight and composition trajectories with prebronchodilation lung function growth rates from age 8 to 15 years. Models are adjusted for maternal social class, maternal smoking during pregnancy, birth weight, breastfeeding, lung function measures at 8 years, pubertal status (age at menarche for girls and voice break status at age 15 yr for boys), and age and height at 15 years. Models for fat mass index and lean body mass index are also mutually adjusted. For definition of abbreviations, see Figure 3.
Increasing LBMI was consistently associated with higher growth rates of FVC, FEV1, and FEF25–75 in both sexes, and this association exhibited a linear dose–response pattern (e.g., in boys included in the “high” LBMI trajectory FVC increased 90.3 ml/yr 95% confidence interval [65.0–115.7] higher than in boys in the “low” LBMI trajectory [P-trend < 0.0001]).
Boys in the “high” (but not “medium-low” or “medium-high”) FMI trajectory exhibited a lower growth rate of FEV1 (−23.2 ml/yr, 95% confidence interval [−40.7 to −5.8]; P value = 0.009) than boys in the “low” FMI and there was a trend toward lower FEF25–75 with higher FMI trajectories (P-trend = 0.045). We did not find any association between FMI trajectories and growth rate of FEV1 or FEF25–75 in girls, nor between FMI and growth rate of FVC in boys or girls.
All sensitivity analyses showed very similar results for LBMI (see Tables E14–E18). For the FMI trajectories, the magnitude of the associations with the growth rate of FEV1 and FEF25–75 (observed only in boys in the main analysis) was attenuated in some of the analyses (see Tables E14–E18) and there was a statistically significant linear trend between FMI trajectories and the growth rate of FVC in girls when we used z-scores (see Table E18).
Discussion
To our knowledge, this is the first study to show that body composition trajectories from childhood to adolescence relate to lung function levels at 15 years and lung function growth rates from age 8 to 15 years in a large population-based birth cohort. Specifically, we found that higher LBMI was associated with higher levels and growth rates of FVC, FEV1, and FEF25–75 in both sexes, and higher FMI was related to lower levels and growth rates of FEV1 and FEF25–75 in boys and to a lower FEV1/FVC ratio in both sexes.
Our finding that a higher lean body mass is related to higher lung function is consistent with observations from previous cross-sectional studies in children and adolescents (9, 14, 15). We show this association longitudinally, reducing the potential for reverse causation, and after adjustment for relevant confounders, such as physical activity, diet, and pubertal status. High lean body mass may reflect increased strength of the diaphragm and chest wall during expansion and contraction during breathing (26), which could produce a greater FVC, FEV1, and FEF25–75 (27). Physical activity (leading to higher levels of lean body mass) (28) could be the ultimate driver of higher lung function measures, but all associations remained stable after adjustment for physical activity (measured by accelerometer). Consequently, other mechanisms are likely to play a role.
Our study is the first to show an association between higher fat mass and increased airflow limitation (as measured by a lower FEV1/FVC ratio) in both sexes. This association is difficult to interpret given the inconsistency of the associations between the fat mass trajectories and each of FEV1 and FVC. Similar inconsistencies have been observed in studies on children that have used BMI as a measure of overweight/obesity; higher BMI seems to be consistently related to a lower FEV1/FVC ratio (4, 7), but the direction of the associations between BMI with FEV1 and FVC varies by study. One explanation could be that the fat mass component is the one that is contributing to the inconsistent results observed for BMI. We also hypothesize a mediating role of inflammation, which could explain the stronger effect of fat mass on airway caliber than on lung capacity. Because adipose tissue is a source of inflammatory mediators (29), local effects of inflammation on lung tissues could lead to reductions in airway diameter. A similar mechanism has also been proposed to explain the link between obesity and asthma (30).
Higher FMI trajectories also were related, only in boys, to lower FEV1 and FEF25–75. A previous cross-sectional study also reported an association between body adiposity (assessed through bioelectrical impedance) and FEV1 and FVC only in boys (15). One explanation could be related to sex differences in fat distribution. Boys, unlike girls, tend to accumulate fat in the abdominal region (31), which via mechanics, may reduce the expiratory reserve volume, in turn leading to expiratory flow limitation (32).
The results of the present study have important research and public health implications. First, our study highlights the importance of assessing body composition, and not just BMI, when studying the health effects of body weight in children and adolescents. Failure to do this has likely contributed to the conflicting findings from multiple studies that have reported associations between overweight/obesity and lung function in children and adolescents (2–8). BMI, a measure based simply on height and total body mass, is unable to distinguish between lean body mass and fat mass, and their relative proportions that vary greatly by age and sex during adolescence as a consequence of puberty (17). In fact, we found important sex differences in the levels and changes over time of lean body mass and fat mass (Figures 1 and 2). Compared with boys, girls had higher levels of fat mass at all ages and showed a higher age-related increase of FMI for all trajectories. In contrast, boys had higher levels of lean body mass at all ages and their age-related increase in LBMI was steeper than in girls. Second, our study shows that body composition in childhood and adolescence influences the development of lung function and, consequently, may affect future respiratory health. Because body composition tracks from childhood to adulthood (17) and is affected by modifiable lifestyle factors, such as physical activity and diet (21, 28, 33), public health strategies promoting healthy lifestyles in early childhood may improve lung function and reduce respiratory morbidity in adult life.
A limitation of the present study is the potential selection bias produced by the fact that children included in the study were more likely to be girls, have a higher socioeconomic status, a higher birth weight, a higher proportion of breastfeeding, and lower maternal smoking exposure than those excluded. Because these factors have been previously associated with lung function, our associations could be underestimates of the true associations in the general population. However, because most of the attrition occurred between birth and age 7 years, the observed associations (which are based largely on data collected from 7 to 15 yr) are less likely to be affected by the loss to follow-up. Also, the regional basis of the ALSPAC cohort may not allow the generalizability of our results to populations with more ethnic variability. Finally, it is possible that using group-based trajectory modeling for identifying trajectories of body weight and composition may have smoothened the data.
Important strengths of the present research are the large sample size and the longitudinal design, which, together with the adjustment for baseline lung function (both for levels of lung function and lung function growth rates), reduces the possibility of reverse causation. Importantly, we measured body composition using dual-energy X-ray absorptiometry, which is substantially more valid than other methods (e.g., bioelectrical impedance or skinfolds). Finally, we had detailed information of several covariates from both the children and their parents, which allowed us to account for a wide range of potential confounders, including physical activity, diet, and baseline lung function.
In conclusion, this cohort study shows that body composition in childhood and adolescence is associated with lung function in adolescence, and consequently, it may also influence respiratory health in later life. Specifically, we found that lean body mass during childhood and adolescence relates to higher lung function in adolescent boys and girls, whereas fat mass relates to lower lung function in boys only. This study shows that public health policies aiming to reduce respiratory morbidity should target body composition in addition to body weight.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all the families who took part in this study; the midwives for their help in recruiting them; and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Footnotes
The present analyses are part of the ALEC (Ageing Lungs in European Cohorts) Study (www.alecstudy.org), which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 633212. The content of this article reflects only the authors’ views, and the European Commission is not liable for any use that may be made of the information contained therein. The UK Medical Research Council and Wellcome Trust (grant reference number: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. A comprehensive list of grant funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). Specifically, grants from Wellcome Trust and Medical Research Council (076467/Z/05/Z and G0401540/73080) supported the collection of body composition and lung function data at 15 years. E.F. is supported by a Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2015; proposal number 704268). C.R. is the recipient of a European Respiratory Society Fellowship (RESPIRE3-201703-00127, under H2020—Marie Skłodowska-Curie Actions COFUND).
Author Contributions: G.P.P. and J.G.-A. prepared the first draft of the paper. G.P.P., I.S., and J.G.-A. had full access to the data and performed statistical analysis. R.G. and J.H. contributed to data collection. J.G.-A. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. G.P.P., E.F., R.G., O.M., C.R., I.S., D.J., J.H., and J.G.-A. provided substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; revised the manuscript for important intellectual content; approved the final version; and agreed to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201806-1168OC on January 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 2.Spathopoulos D, Paraskakis E, Trypsianis G, Tsalkidis A, Arvanitidou V, Emporiadou M, et al. The effect of obesity on pulmonary lung function of school aged children in Greece. Pediatr Pulmonol. 2009;44:273–280. doi: 10.1002/ppul.20995. [DOI] [PubMed] [Google Scholar]
- 3.Davidson WJ, Mackenzie-Rife KA, Witmans MB, Montgomery MD, Ball GDC, Egbogah S, et al. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. 2014;49:1003–1010. doi: 10.1002/ppul.22915. [DOI] [PubMed] [Google Scholar]
- 4.Forno E, Han Y-Y, Mullen J, Celedón JC. Overweight, obesity, and lung function in children and adults: a meta-analysis. J Allergy Clin Immunol Pract. 2018;6:570–581. doi: 10.1016/j.jaip.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekkers MB, Wijga AH, Gehring U, Koppelman GH, de Jongste JC, Smit HA, et al. BMI, waist circumference at 8 and 12 years of age and FVC and FEV1 at 12 years of age; the PIAMA birth cohort study. BMC Pulm Med. 2015;15:39. doi: 10.1186/s12890-015-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58:1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekström S, Hallberg J, Kull I, Protudjer JLP, Thunqvist P, Bottai M, et al. Body mass index status and peripheral airway obstruction in school-age children: a population-based cohort study. Thorax. 2018;73:538–545. doi: 10.1136/thoraxjnl-2017-210716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suresh S, O’Callaghan M, Sly PD, Mamun AA. Impact of childhood anthropometry trends on adult lung function. Chest. 2015;147:1118–1126. doi: 10.1378/chest.14-0698. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Barcala FJ, Takkouche B, Valdes L, Leis R, Alvarez-Calderon P, Cabanas R, et al. Body composition and respiratory function in healthy non-obese children. Pediatr Int. 2007;49:553–557. doi: 10.1111/j.1442-200X.2007.02420.x. [DOI] [PubMed] [Google Scholar]
- 10.Kongkiattikul L, Sritippayawan S, Chomtho S, Deerojanawong J, Prapphal N. Relationship between obesity indices and pulmonary function parameters in obese Thai children and adolescents. Indian J Pediatr. 2015;82:1112–1116. doi: 10.1007/s12098-015-1777-4. [DOI] [PubMed] [Google Scholar]
- 11.Williams JE, Wells JCK, Benden C, Jaffe A, Suri R, Wilson CM, et al. Body composition assessed by the 4-component model and association with lung function in 6-12-y-old children with cystic fibrosis. Am J Clin Nutr. 2010;92:1332–1343. doi: 10.3945/ajcn.2010.29847. [DOI] [PubMed] [Google Scholar]
- 12.Pedreira CC, Robert RGD, Dalton V, Oliver MR, Carlin JB, Robinson P, et al. Association of body composition and lung function in children with cystic fibrosis. Pediatr Pulmonol. 2005;39:276–280. doi: 10.1002/ppul.20162. [DOI] [PubMed] [Google Scholar]
- 13.Li AM, Chan D, Wong E, Yin J, Nelson EAS, Fok T. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88:361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen ME, Gibson PG, Collins CE, Wood LG. Lean mass, not fat mass, is associated with lung function in male and female children with asthma. Pediatr Res. 2014;75:93–98. doi: 10.1038/pr.2013.181. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Custovic A, Simpson A, Belgrave DC, Lowe LA, Murray CS. Differing associations of BMI and body fat with asthma and lung function in children. Pediatr Pulmonol. 2014;49:1049–1057. doi: 10.1002/ppul.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazarus R, Colditz G, Berkey CS, Speizer FE. Effects of body fat on ventilatory function in children and adolescents: cross-sectional findings from a random population sample of school children. Pediatr Pulmonol. 1997;24:187–194. doi: 10.1002/(sici)1099-0496(199709)24:3<187::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Chumlea WC, Siervogel RM. Age and maturity related changes in body composition during adolescence into adulthood: the Fels longitudinal study. Int J Obes. 1997;21:1167–1175. doi: 10.1038/sj.ijo.0800531. [DOI] [PubMed] [Google Scholar]
- 18.Peralta GP, Fuertes E, Garcia-Aymerich J, Henderson J, Jarvis D.Lean body mass is positively associated with lung function at age 15. Presented at the European Respiratory Society Congress. September 9–13, 2017, Milan, Italy [Google Scholar]
- 19.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children. ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddoch CJ, Leary SD, Ness AR, Blair SN, Deere K, Mattocks C, et al. Prospective associations between objective measures of physical activity and fat mass in 12-14 year old children: the Avon Longitudinal Study of Parents and Children (ALSPAC) BMJ. 2009;339:b4544. doi: 10.1136/bmj.b4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Standardization of spirometry, 1994 update: American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 23.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. BMI-for-age (5–19 years); 2015 [accessed 2017 Mar 23]. Available from: http://www.who.int/growthref/who2007_bmi_for_age/en/
- 25.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura Y, Tsutsumi M, Nakata H, Tsunenari T, Maeda H, Yokoyama M. Relationship between respiratory muscle strength and lean body mass in men with COPD. Chest. 1995;107:1232–1236. doi: 10.1378/chest.107.5.1232. [DOI] [PubMed] [Google Scholar]
- 27.Bae JY, Jang KS, Kang S, Han DH, Yang W, Shin KO. Correlation between basic physical fitness and pulmonary function in Korean children and adolescents: a cross-sectional survey. J Phys Ther Sci. 2015;27:2687–2692. doi: 10.1589/jpts.27.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez-Pavon D, Fernandez-Vazquez A, Alexy U, Pedrero R, Cuenca-Garcia M, Polito A, et al. HELENA Study Group. Association of objectively measured physical activity with body components in European adolescents. BMC Public Health. 2013;13:667. doi: 10.1186/1471-2458-13-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 30.Boulet LP. Asthma and obesity. Clin Exp Allergy. 2013;43:8–21. doi: 10.1111/j.1365-2222.2012.04040.x. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RW, Grant AM, Williams SM, Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity (Silver Spring) 2010;18:1410–1416. doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 32.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 33.Smith ADAC, Emmett PM, Newby PK, Northstone K. Dietary patterns and changes in body composition in children between 9 and 11 years. Food Nutr Res. 2014;58:22769. doi: 10.3402/fnr.v58.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.