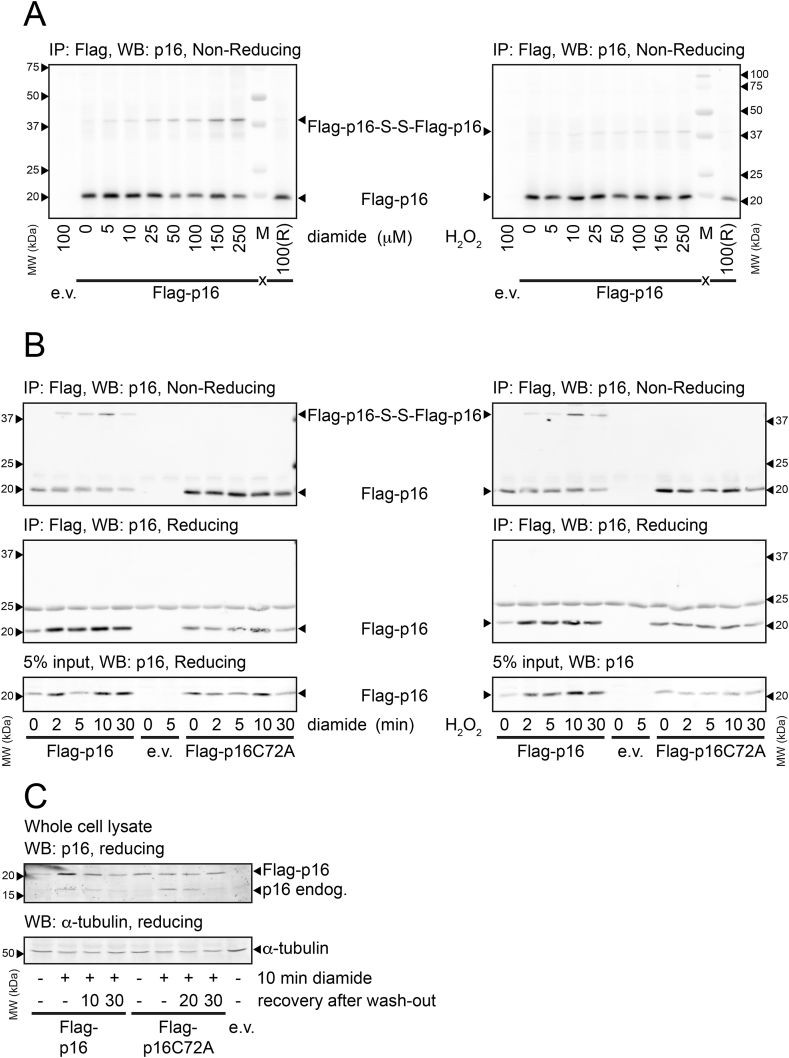
Fig. 1.
p16INK4Aforms intermolecular disulfides upon exposure to oxidants.
(A) Analysis of Immuno-precipitated FLAG-p16INK4A by non-reducing SDS-PAGE and Western Blot shows that part of the FLAG-p16INK4A migrates at about double the molecular weight upon 5 min treatment with low amounts of the thiol-specific oxidant diamide (left panel) or H2O2 (right panel). Reduction (R) prior to SDS-PAGE abolishes the shift in molecular weight, indicating that it is indeed due to an intermolecular disulfide (see also Figs. S2A and S2B for confirmation that the high molecular weight form of p16INK4A is an S–S-dependent homodimer).
(B) S–S-dependent p16INK4A homodimerization upon 200 μM diamide (left) or 200 μM H2O2 (right) occurs rapidly, coincides with accumulation of p16INK4A protein levels and oxidation as well as accumulation are fully dependent on C72.
(C) Endogenous p16INK4A and over-expressed FLAG-p16INK4A accumulate in response to 200 μM diamide with similar kinetics. Note that p16INK4AC72A does not accumulate whereas endogenous p16INK4A does, suggesting that endogenous p16INK4A levels are also regulated by Cys-oxidation. (IP: immunoprecipitation, WB: Western Blot). All Western blots shown in Fig. 1 are typical results of several repeats (N ≥ 3 for all experiments).