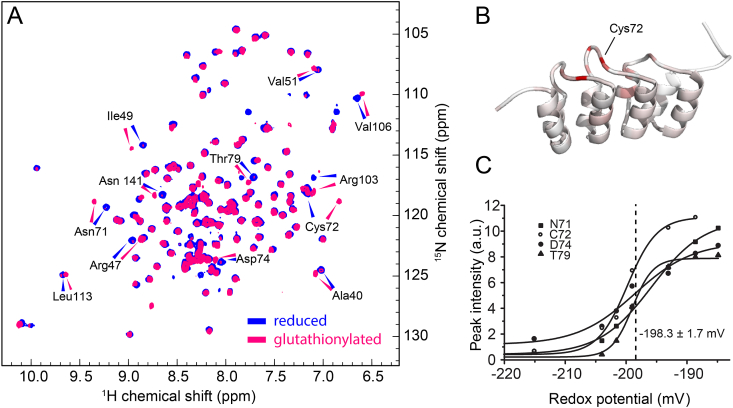
Fig. 2.
In vitrooxidation of p16INK4A
(A)1H15N HSQC solution NMR spectrum of recombinant p16INK4A in the reduced state (blue) and after S-glutathionylation (magenta). Amino acids with large chemical shift changes are labeled.
(B) Cartoon representation of the p16INK4A structure. A color gradient from white (unaffected) to red (strongly affected) shows the influence of S-glutathionylation on the chemical shift.
(C) The redox potential of C72 is 198.3 ± 1.7 mV, as determined from intensity changes of four well-separated amino acids by titration of the reduced protein with oxidized glutathione. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)