Abstract
Background: Pulmonary complications of sickle cell disease (SCD) are diverse and encompass acute and chronic disease. The understanding of the natural history of pulmonary complications of SCD is limited, no specific therapies exist, and these complications are a primary cause of morbidity and mortality.
Methods: We gathered a multidisciplinary group of pediatric and adult hematologists, pulmonologists, and emergency medicine physicians with expertise in SCD-related lung disease along with an SCD patient advocate for an American Thoracic Society–sponsored workshop to review the literature and identify key unanswered clinical and research questions. Participants were divided into four subcommittees on the basis of expertise: 1) acute chest syndrome, 2) lower airways disease and pulmonary function, 3) sleep-disordered breathing and hypoxia, and 4) pulmonary vascular complications of SCD. Before the workshop, a comprehensive literature review of each subtopic was conducted. Clinically important questions were developed after literature review and were finalized by group discussion and consensus.
Results: Current knowledge is based on small, predominantly observational studies, few multicenter longitudinal studies, and even fewer high-quality interventional trials specifically targeting the pulmonary complications of SCD. Each subcommittee identified the three or four most important unanswered questions in their topic area for researchers to direct the next steps of clinical investigation.
Conclusions: Important and clinically relevant questions regarding sickle cell lung disease remain unanswered. High-quality, multicenter, longitudinal studies and randomized clinical trials designed and implemented by teams of multidisciplinary clinician-investigators are needed to improve the care of individuals with SCD.
Keywords: sickle cell disease, acute chest syndrome, asthma, sleep disorders, pulmonary hypertension
Contents
Introduction
Methods
Committee Composition
Subcommittees
Literature Review
Workshop Overview
Document Preparation
Results
Understanding the Natural History of Sickle Cell Lung Disease Is Critical
ACS
ACS Severity and Subtypes
Primary and Secondary Prevention of ACS
Management of ACS in Low-Resource Settings
Lower Airway Disease and Pulmonary Function
Lung Function across the Lifespan
Pathophysiology of Lower Airway Disease
Impact of Lower Airway Disease on SCD Outcomes
SDB and Hypoxemia
Assessment of Hypoxemia and SDB
Consequences of SDB and Recurrent Oxygen Desaturation
Treatment of SDB and Recurrent Oxygen Desaturation
Pulmonary Vascular Complications of SCD
Impact of PH Diagnosis on Outcomes
Treatment of SCD-related PAH
Deep Venous Thrombosis Prophylaxis in Specific Cohorts of Patients with SCD
Secondary Prevention of DVT, Pulmonary Embolism, or Pulmonary Artery Thrombosis
Future Directions
Summary
Introduction
Sickle cell disease (SCD) is a genetic hemoglobinopathy associated with early mortality that occurs in racial and ethnic groups that are profoundly impacted by social determinants of health and commonly have poor access to health care (1). Although mortality for children living with SCD in high-resource settings has improved, pulmonary-related morbidity remains a high burden across the lifespan. These factors and others have led to significant health disparities and contributed to our limited understanding of disease progression and its complications. In both prospective and retrospective studies, pulmonary complications, implicating nearly every cell type and structure of the lung, continue to be the most common etiology of accelerated morbidity and mortality in individuals living with SCD (2–8). Existing National Heart Lung and Blood Institute (NHLBI) guidelines were developed to aid primary care providers with the management of SCD; however, a lack of high-quality data limited their usefulness in addressing sickle cell lung disease (9). Given that cardiopulmonary complications of SCD are a major risk factor for death in this population, expanding our knowledge of lung disease is crucial to improving patient outcomes.
Fundamental challenges in addressing sickle cell lung disease include a limited understanding of: 1) the natural history of SCD-associated pulmonary disease, 2) genetic factors and comorbidities that increase pulmonary risk in SCD, and, consequently, 3) a lack of SCD-specific therapies. To address these challenges and frame the research agenda for the next 5 to 10 years, we gathered an expert panel for an American Thoracic Society (ATS) workshop to identify key unanswered research questions in the following topics:
-
1.
Acute chest syndrome (ACS)
-
2.
Lower airways disease and pulmonary function
-
3.
Sleep-disordered breathing (SDB) and hypoxemia
-
4.
Pulmonary vascular complications in SCD
This ATS Workshop Report serves to disseminate our findings to the medical community. The key unanswered research questions identified by the Workshop Committee are listed in Table 1.
Table 1.
Summary of key unanswered research questions
| Research Topics | Key Unanswered Clinical and Research Questions |
|---|---|
| ACS | |
| ACS severity and subtypes | Which criteria should be used to classify ACS as mild, moderate, or severe? |
| Which tests are best for determining specific etiologies of ACS? | |
| Which biomarkers reliably predict ACS severity? | |
| What therapies improve ACS outcomes? | |
| Primary and secondary prevention of ACS | Which patients are at increased risk for ACS and may benefit from primary or secondary prevention? |
| Can we identify patients with vaso-occlusive events are at highest risk of ACS? | |
| Which interventions are effective in preventing first-time and recurrent ACS? | |
| Management of ACS in low-resource settings | How should patients with acute onset of signs and symptoms concerning for ACS be managed in low-resource settings? |
| Which physical signs/symptoms are most indicative of ACS in settings without chest radiography? | |
| Which sustainable interventions decrease ACS-related maternal mortality in low-resource settings where hematologists and transfusion therapy are not uniformly available? | |
| Lower airway disease and pulmonary function | |
| Lung function across the lifespan | What are the characteristics of lung function in SCD patients across the lifespan? |
| Is there a predominant lung function pattern in SCD? | |
| Do lung function patterns evolve in patients with SCD across the lifespan? | |
| Pathophysiology of lower airway disease | What are the features and pathophysiology of lower airway disease in SCD? |
| Which inflammatory pathways contribute to lower airway disease? | |
| How do pulmonary vascular abnormalities contribute to lower airway disease? | |
| Impact of lower airway disease on SCD outcomes | Does lower airway disease impact clinical outcomes in SCD? |
| What is the relationship between lower airway disease and SCD outcomes? | |
| Is there a role for screening asymptomatic patients with PFTs? | |
| Are there modifiable risk factors for lower airway disease? | |
| SDB and hypoxemia in SCD | |
| Assessment of hypoxemia and SDB | What are the optimal approaches to evaluating hypoxemia, oxygen desaturation, and SDB? |
| Which signs and symptoms are useful to identify individuals who warrant formal evaluation for nocturnal respiratory disorders? | |
| Which alternative to full, in-laboratory polysomnography could be used to identify SDB and nocturnal hypoxemia in individuals with SCD? | |
| Consequences of SDB and recurrent oxygen desaturation | How do OSA, sustained versus intermittent oxygen desaturation, and repeated arousals during sleep impact SCD morbidity and mortality? |
| Which oxygen saturation threshold for nocturnal hypoxemia contributes to SCD morbidity and mortality? | |
| Treatment of SDB and recurrent oxygen desaturation | What is the optimal treatment of SDB among individuals with SCD? |
| What is the acceptability of treatment for hypoxemia and SDB? | |
| What is the impact of treatment of hypoxemia and SDB on short- and long-term outcomes in SCD? | |
| Pulmonary vascular complications of SCD | |
| Impact of PH diagnosis on outcomes | Does the early identification and treatment of PH in SCD improve outcomes? |
| In the asymptomatic patient, does evaluation for SCD-PH impact clinical outcomes? | |
| What is the best strategy to screen for SCD-PH? | |
| What should be the approach to abnormal screening studies or the diagnosis of the symptomatic patient? | |
| Treatment of SCD-related PAH | Do patients with SCD-PAH respond to PAH therapy? |
| Which criteria should be used to determine which patients should receive PAH therapy? | |
| Which novel PAH therapeutics hold the most promise for patients with SCD-PAH? | |
| What should be the treatment approach for patients with PH related to left-sided heart disease? | |
| DVT prophylaxis in specific cohorts of patients with SCD | How should DVT prophylaxis be approached in pregnant women and children with SCD? |
| Secondary prevention of DVT, PE, or pulmonary artery thrombosis | If a patient with SCD is diagnosed with a DVT, PE, or pulmonary artery thrombosis, what treatment should be used and for what duration? |
| What are indications for long-term anticoagulation in SCD? | |
Definition of abbreviations: ACS = acute chest syndrome; DVT = deep venous thrombosis; OSA = obstructive sleep apnea; PAH = pulmonary arterial hypertension; PE = pulmonary embolism; PFT = pulmonary function test; PH = pulmonary hypertension; SCD = sickle cell disease; SDB = sleep-disordered breathing.
Methods
Here we describe the methods of the workshop committee composition, literature review, and manuscript preparation (Table 2).
Table 2.
Summary of workshop methods
| Proposed Methods Checklist: for Each, Respond as Yes or No | Yes | No |
|---|---|---|
| Panel assembly: | ||
| Included experts from all relevant clinical and nonclinical disciplines | X | |
| Included individual who represents views of patients and society at large | X | |
| Included methodologist with appropriate expertise (documented expertise in development of conducting systematic reviews to identify the evidence base and development of evidence-based recommendations). | X | |
| Literature review: | ||
| Performed in collaboration with librarian | X | |
| Searched multiple electronic databases | X | |
| Reviewed reference lists of retrieved articles | X | |
| Evidence synthesis: | ||
| Applied prespecified inclusion and exclusion criteria | X | |
| Evaluated included studies for sources of bias | X | |
| Explicitly summarized benefits and harms | X | |
| Used GRADE to describe quality of evidence | X |
Definition of abbreviation: GRADE = Grading of Recommendations Assessment, Development, and Evaluation.
Committee Composition
The committee included SCD experts in adult and pediatric hematology, pulmonology, sleep medicine, and emergency medicine, and one patient advocate. The project chairs, adult (A.P.R. and E.S.K.) and pediatric (S.C.S. and R.T.C.) pulmonologists, selected committee members on the basis of their expertise. The committee consisted of 32 physicians (including the four chairs) from the United States, United Kingdom, Jamaica, and Mali (6 adult pulmonologists, 6 adult hematologists, 12 pediatric pulmonologists, 6 pediatric hematologists and 2 emergency medicine physicians [1 adult and 1 pediatric]), one librarian, and one patient representative with SCD. Two of the pediatric pulmonologists were board certified in sleep medicine. The committee was divided into four subcommittees, each led by a workshop co-chair, to address the key topics. All committee members disclosed their potential conflicts of interest to the ATS and the project chairs, who reviewed them, discussed them with the chair of the Ethics and Conflict of Interest Committee of the ATS, and resolved them with the individual committee members. Committee members were required to refrain from discussing topics related to their conflicts of interest.
Subcommittees
Four subcommittees were formed to focus the workshop on the following topics: 1) Acute chest syndrome (A.P.R., D.A.D., J.A.G., J.H., J.K.-M., C.R.M., S.L.T., E.V.), 2) Lower airways disease and pulmonary function (S.C.S., J.J.F., L.M.G., A.G., B.T.K., A.C.K., R.I.L., F.O.O., K.S.-W.), 3) SDB and hypoxia (J.L.A., A.D.C., T.D.C., E.K.F., C.L.R., D.T., R.T.C.), 4) Pulmonary vascular complications of SCD (D.P.B., M.T.G., V.R.G., G.J.K., S.M.L., R.F.M., A.M., N.A.W., E.S.K.). Each subcommittee, led by one of the co-chairs, held a conference call before the all-day meeting at the 2018 ATS International Conference for identification of important potential areas of focus for the workshop.
Literature Review
A comprehensive literature search was performed on each topic before the workshop by a librarian from the National Institutes of Health Library (N.T.) and was updated during the preparation of this report. Common search parameters used for each topic included SCD and human subjects research in both children and adults in the PubMed and Cochrane Library databases from 1970 to January 28th, 2019. Additional literature was cited as it became available.
Workshop Overview
Each chair presented an overview of the current state of knowledge of their respective topic followed by a group discussion with all workshop participants. This was followed by breakout subcommittee meetings, where the most important questions in each topic were identified. The workshop concluded with each subcommittee presenting their findings to the entire group.
Document Preparation
After the workshop, each subcommittee analyzed and synthesized the evidence for their topic, applied it to the broader context of the field, and drafted a section of the document. These drafts were edited by the co-chairs, and the document was distributed for discussion and editing. All committee members had the opportunity to contribute to the document. The key unanswered research questions are listed in Table 1.
Results
Understanding the Natural History of Sickle Cell Lung Disease Is Critical
At the workshop, a common theme unified all discussions: there are significant limitations in our knowledge because of a relatively poor understanding of the natural history of sickle cell lung disease and a lack of data, in most cases, on the clinical significance of pulmonary comorbidities. The current situation is a result of several missed opportunities.
Although every major study of SCD has identified cardiopulmonary disease as the major cause of death (10), current knowledge has been hindered by a lack of prospective data on the natural history of sickle cell lung disease and a lack of a widely accessible standardized SCD registry that would provide these data for both high- and low-resource settings (3).
The Cooperative Study of Sickle Cell Disease, a prospective clinical study conducted between 1978 and 1998 across 23 centers in the United States, was intended to set a framework for understanding SCD and its multisystem complications. Unfortunately, the assessment of chronic cardiopulmonary disease was limited by incomplete collection of longitudinal pulmonary function data and a lack of ascertainment of complications such as pulmonary hypertension (PH) and SDB. Furthermore, patients were enrolled in 1979 to 1981 (11), before the widespread use of penicillin prophylaxis, pneumococcal vaccination, and hydroxyurea, limiting the applicability of findings to the current SCD population. More recently, most pulmonary-focused studies in this underserved and understudied population have been small, retrospective, and focused either on pediatric or adult populations, but not both. There have been no studies on progression of pulmonary disease in the “transition age group” from late adolescence through early adulthood when the care of patients transfers from pediatric to adult providers, patients assume responsibility for their own care, and, importantly, functional capacity and quality of life drop precipitously, with an increase in mortality (12). Consequently, although we understand that children and adolescents in settings with access to preventive SCD care have benefitted from improved mortality over the last several decades, they continue to suffer significant morbidity from sickle cell lung disease, while adults continue to suffer from high mortality even in high-resource settings.
In terms of lung function, we know that individuals with SCD have lower lung function than those without SCD. About 15% to 20% of children and adolescents have obstructive physiology; by adulthood (13, 14), many individuals meet criteria for restrictive disease (15, 16), yet we do not understand the evolution of lung function, the clinical significance of these abnormalities, or how they may be related. Similarly, increased tricuspid regurgitant jet velocity (TRV) observed in 10% to 20% of children and adolescents with SCD has unclear prognostic significance (17, 18), whereas in adults these echocardiographic findings have been repeatedly associated with early mortality (8, 19, 20). The workshop committee strongly advocates for a modern-day, prospective, longitudinal multicenter assessment of the pulmonary complications of SCD encompassing pediatric and adult populations. The workshop committee also strongly acknowledges the need to include high-prevalence areas of SCD, such as sub-Saharan Africa, the Middle East, India, and Brazil, in clinical research studies (21).
Each of the four subcommittees identified the priority focus areas for their topic. The results of these discussions are presented here.
ACS
ACS is a clinical syndrome consisting of chest pain, fever, tachypnea, wheezing, rales, or cough plus a new infiltrate involving at least one lung segment (22–24) and is a leading acute cause of mortality in this population (24). This nonspecific definition applies to episodes with variable etiologies, severities, and timing of symptom onset (24–26). Diagnosis relies on radiographic abnormalities, which may precede or lag behind symptoms, and radiography may be unavailable in low-resource settings. Although one randomized clinical trial demonstrated that incentive spirometry during vaso-occlusive crisis decreases ACS risk (27), additional preventive approaches are necessary. It is possible that modern informatics and machine learning could use large clinical data sets to identify other preventive therapies, risk factors, or early indicators of ACS, such as a decrease in oxygen saturation (28). The need to perform clinical research in ACS in international areas of high SCD prevalence is pressing, as improved understanding of these populations is vital to advance our ability to diagnose, prevent, and treat ACS in low-resource settings.
A comprehensive understanding of ACS pathogenesis is needed to evaluate the efficacy of existing therapies and develop preventive strategies and targeted treatments (9). Priority areas for research include: 1) ACS severity and subtypes, 2) primary and secondary prevention, and 3) management in low-resource settings.
ACS Severity and Subtypes
Research question: Which criteria should be used to classify ACS as mild, moderate, or severe?
Supporting questions:
-
•
Which tests are best for determining specific etiologies of ACS?
-
•
Which biomarkers reliably predict ACS severity?
-
•
What therapies improve ACS outcomes?
The umbrella term “ACS” describes a clinical syndrome with distinct phenotypes that require better characterization. Rapidly progressive ACS, associated with multiorgan failure and death (2), appears distinct from milder ACS with minimal respiratory signs and symptoms and improves with antibiotics and supportive care. Some ACS episodes are associated with transient or worsening PH (29), whereas others (particularly in children) mimic an asthma exacerbation (30). Designing therapeutic trials targeting ACS is challenging, given the diverse underlying etiologies of this syndrome (i.e., infection, bone marrow/fat embolism, thrombosis) (24).
Development of an ACS risk and severity stratification algorithm is a top research priority for clinical trial development. Currently, the criteria used to define severity are applied after the ACS course has evolved. In comparison, timing of symptom onset was integrated into the 2012 Berlin definition of acute respiratory distress syndrome (ARDS) allowing for risk prediction. A recent retrospective study of 173 children and adults with SCD found that, in adults, thrombocytopenia was the only predictor of rapidly progressive ACS (2). This needs to be confirmed prospectively in a larger cohort, as these patients would require immediate, aggressive therapy if at risk for severe morbidity and mortality (2, 31). Additional biomarkers (32–34) should be evaluated for their potential in predicting ACS development and progression, including: plasma free hemoglobin, soluble phospholipase A2 (32), and circulating exosomes (33), and also common laboratory tests such as white blood cell count, nucleated red blood cells, or C-reactive protein (34). Other experimental biomarkers, such as soluble phospholipase A2 in exhaled breath condensate, are currently under study (35). Much like updates providing clarity for definition of ARDS (36), ACS phenotype severity classifications should be explicitly defined and categorized by: 1) acuity and severity, and 2) identifiable pathophysiology allowing for targeted therapies.
Primary and Secondary Prevention of ACS
Research question: Which patients are at increased risk for ACS and may benefit from primary or secondary prevention?
Supporting questions:
-
•
Can we identify which patients with vaso-occlusive events (VOEs) are at highest risk of ACS?
-
•
Which interventions are effective in preventing first-time and recurrent ACS?
ACS often occurs 1 to 3 days after hospital admission for VOEs, but patients can also present with ACS independently of a painful episode (24, 37, 38). Hydroxyurea, l-glutamine, and chronic transfusions decrease ACS frequency but do not prevent all episodes (39, 40). Some clinicians use early blood transfusion or noninvasive ventilation (NIV) in patients at high risk for ACS. Ideal timing and type of transfusion (simple vs. exchange) and the efficacy of NIV to decrease ACS progression should be tested in intervention trials. Systemic steroids may reduce the severity and/or duration of an ACS episode, but some studies and anecdotal observations suggest an increased risk of rehospitalization for a VOE (41–45).
Pharmacologic therapy for ACS prevention (inhaled corticosteroids [46, 47] and novel medications including crizanlizumab, a monoclonal antibody targeting P-selectin [48]) should be evaluated in large randomized trials (15). Agents targeting erythrocyte sickling, hemolysis, fat embolism, and thrombosis should be investigated (49). Moreover, preventive measures against ARDS should be evaluated as preventive measures for ACS in patients with SCD, such as low–tidal-volume ventilation, either in the intensive care unit or when undergoing general anesthesia.
Management of ACS in Low-Resource Settings
Research question: How should patients with acute onset of signs and symptoms concerning for ACS be managed in low-resource settings?
Supporting questions:
-
•
Which physical signs/symptoms are most indicative of ACS in settings without chest radiography?
-
•
Which sustainable interventions decrease ACS-related maternal mortality in low-resource settings where hematologists and transfusion therapy are not uniformly available?
ACS is a common cause of hospitalization in SCD in high-resource settings (24, 37), but in many low-resource regions where SCD is highly prevalent, chest radiographs are unavailable or cost prohibitive, creating diagnostic challenges (50). A diagnostic strategy for ACS independent of imaging could facilitate early identification and intervention allowing for targeted resource use. The role of portable pulse oximetry devices in ACS diagnosis should be evaluated.
Pregnancy and the postpartum period are associated with increased ACS risk. ACS is the most common cause of acute respiratory failure and death in pregnant or postpartum SCD patients in low-resource settings, with ACS responsible for >60% of the deaths in pregnant women with SCD in this population (31, 51). In Ghana, a multidisciplinary approach using a combined obstetric and hematology team was effective in reducing ACS incidence and all-cause maternal mortality (52). This suggests that standardized early identification and management protocols for pregnant and postpartum patients with SCD could improve both maternal and fetal outcomes.
Lower Airway Disease and Pulmonary Function
Many children and adults with SCD have abnormal pulmonary function and/or recurrent respiratory symptoms. NHLBI SCD management guidelines recommend against screening pulmonary function tests (PFTs) (53); however, a better understanding of the pathophysiology and progression of lower airway disease is needed to understand the potential benefits of screening. Priority areas for research include: 1) lung function across the lifespan, 2) pathophysiology of lower airway disease, and 3) the impact of lower airway disease on SCD outcomes.
Lung Function across the Lifespan
Research question: What are the characteristics of lung function in patients with SCD across the lifespan?
Supporting questions:
-
•
Is there a predominant lung function pattern in SCD?
-
•
Do lung function patterns evolve in patients with SCD across the lifespan?
Studies demonstrate PFT abnormalities in infants (54), children (7, 55–58), and adults (15, 16, 59). Children, on average, have reduced lung function compared with control subjects, but most are still within the normal range. Obstructive physiology is the most common abnormality in children (13, 14), whereas many adults have restrictive physiology (15, 16, 60). It is not clear whether the obstructive defects observed in childhood lead to restrictive defects in adulthood (i.e., if there is a progression or trajectory of PFT abnormalities), or whether these abnormalities affect different patient subgroups at different points in their lifespan.
Our understanding of lung function in SCD has been impaired by inconsistent study design, nonuniform interpretation/classification strategies when comparing results across studies, and a lack of longitudinal data (61). Use of spirometry alone overestimates the prevalence of restrictive defects, and obstruction may be underestimated when absolute forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) cutoffs are used. There are few longitudinal lung function studies in SCD, and most were retrospective. One pediatric cohort demonstrated a 2% to 3% annual decline in FVC, FEV1, and total lung capacity (62); however, this finding has not been replicated in other studies (63, 64).
Beyond the increased mortality risk associated with a reduced FEV1 (6, 65), the significance of abnormal PFTs in SCD remains unknown. There is no association between abnormal lung function and rates of pain or ACS in children (13), but recurrent ACS episodes may be associated with reduced total lung capacity in adults (60). Future research should examine associations between abnormal lung function, respiratory symptoms, and SCD outcomes beyond rates of VOEs and ACS and, most importantly, how evaluation of lung function can aid providers in optimizing the care of their patients with cardiopulmonary symptoms (66).
Pathophysiology of Lower Airway Disease
Research question: What are the features and pathophysiology of lower airway disease in SCD?
Supporting questions:
-
•
Which inflammatory pathways contribute to lower airway disease?
-
•
How do pulmonary vascular abnormalities contribute to lower airway disease?
The prevalence of physician-diagnosed asthma among children with SCD is approximately 25% (67). However, many more patients exhibit isolated recurrent wheezing (68, 69), lower airway obstruction (13, 14, 70), or airway hyperresponsiveness (AHR) (71–73) without meeting diagnostic criteria for asthma. Distinguishing between wheezing due to comorbid asthma versus SCD-specific mechanisms is important for delineating pathogenesis and guiding therapeutics (74). Activated neutrophils (75) and fibrocytes (76, 77), proinflammatory cytokines (78), exaggerated allergic inflammation (71, 79–82), increased placental growth factor (83, 84), and increased pulmonary vascular congestion have been implicated in the pathogenesis of airway disease in SCD (59, 85, 86). Future research should focus on elucidating these mechanisms further to identify novel therapeutic approaches.
Impact of Lower Airway Disease on SCD Outcomes
Research question: Does lower airway disease impact clinical outcomes in SCD?
Supporting questions:
-
•
What is the relationship between lower airway disease and SCD outcomes?
-
•
Is there a role for screening asymptomatic patients with PFTs?
-
•
Are there modifiable risk factors for lower airway disease?
Childhood asthma is associated with more frequent VOEs, earlier onset of ACS, recurrent ACS, and mortality (67, 87, 88). In one adult cohort, patient-reported “recurrent wheezing” (but not asthma) was associated with increased morbidity and mortality (68). Other features of airway dysfunction, including AHR and lower airway obstruction, have not been consistently associated with SCD morbidity (13, 89). Environmental exposures, including outdoor air pollution (90, 91) and tobacco smoke (92–96), may contribute to SCD morbidity via direct or indirect effects on airways or pulmonary vasculature, but more study is needed.
Little is known about the impact of treating lower airway disease in SCD. Although one retrospective study demonstrated that hydroxyurea was associated with slower longitudinal lung function decline (97), the impact of hydroxyurea on AHR and wheezing is unknown. A randomized pilot trial of inhaled corticosteroids in adults with SCD without asthma with recent respiratory symptoms found decreased soluble vascular cell adhesion molecule-1 levels (suggesting reduced inflammation and cellular adhesion) and reduced daily pain scores in the inhaled steroid group compared with the placebo group (98). These studies support overlap between lower airway disease and SCD pathobiology.
Determining the impact of antiinflammatory pulmonary treatment on SCD morbidity and mortality is a priority. Similarly, the impact of SCD disease-modifying therapy on lower airway disease needs further evaluation. Asthma therapy should be studied specifically in patients with SCD, because unique toxicities may be observed in this population. For example, systemic steroids have been shown to be beneficial in numerous acute pulmonary conditions including asthma exacerbations in the general population, but, as described above, steroids may increase the risk of rebound pain or avascular necrosis in SCD (41, 42).
SDB and Hypoxemia
SDB, hypoxemia, and other sleep disturbances appear to be common in SCD and may impact SCD morbidity and mortality (99). SDB includes obstructive sleep apnea (OSA), central sleep apnea, and sleep-related hypoventilation. Individuals may separately have sustained (diurnal), intermittent, or sustained nocturnal hypoxemia. Adults with SCD may be at risk for central sleep apnea secondary to chronic opioid use and/or comorbid congestive heart failure (100).
Insufficient and inconsistent data concerning SDB and hypoxemia in SCD led the NHLBI Guidelines for Management of SCD committee to recommend screening for OSA symptoms only, even though some patients with SCD have SDB without typical symptoms (101, 102). Hydroxyurea and blood transfusions improve oxygen saturation (103), suggesting that increasing hemoglobin concentration can improve tissue oxygen delivery. Priority areas for research include: 1) assessment of blood and tissue oxygen content and SDB, 2) consequences of SDB and episodic versus sustained oxygen desaturation, and 3) impact of treating SDB and oxygen desaturation.
Assessment of Hypoxemia and SDB
Research question: What are the optimal approaches to evaluating hypoxemia, oxygen desaturation, and SDB?
Supporting questions:
-
•
Which signs and symptoms are useful to identify individuals who warrant evaluation for nocturnal respiratory disorders?
-
•
Which alternatives to full, in-laboratory polysomnography (PSG) could be used to identify SDB and nocturnal hypoxemia in individuals with SCD?
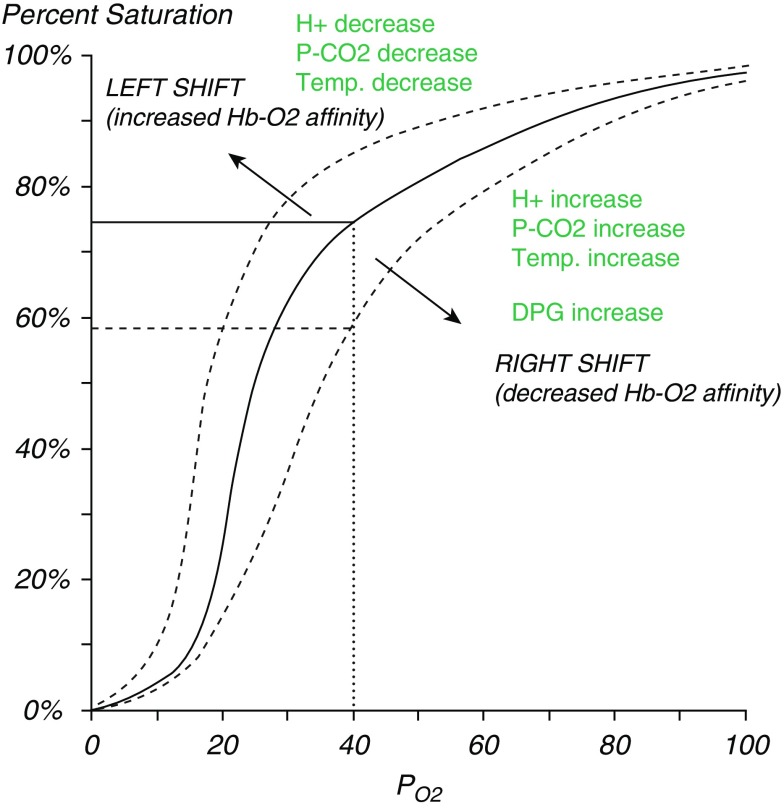
The gold standard for measuring blood oxygen content is an arterial blood gas with CO-oximetry that measures carboxyhemoglobin and methemoglobin. CO-oximetry is important in SCD because it accounts for hemolysis-related dyshemoglobinemia (104, 105). Pulse oximetry is a noninvasive modality to measure oxyhemoglobin saturation (106); however, the accuracy of pulse oximetry in patients with SCD, particularly during VOEs and/or severe anemia, has been questioned (107) because of: 1) rightward shifts of the oxyhemoglobin dissociation curve (Figure 1) (108), and 2) dyshemoglobins, which absorb light at the two wavelengths analyzed by pulse oximetry and may confound measurements (109). There is controversy concerning the appropriate normal oxygen saturation range in SCD. The rightward shift of the oxyhemoglobin dissociation curve in the setting of severe hemolytic anemia suggests that oxygen saturation targets appropriate for the general population may not be applicable for all individuals with SCD (110). Right-to-left shunting (either intracardiac or intrapulmonary) has recently been identified as a possible etiology for oxygen desaturation in a subset of patients (111, 112). However, data suggest that the normal range of oxygen saturation for patients with SCD should be similar to that of healthy control subjects (28, 113).
Figure 1.
Oxyhemoglobin dissociation curve. Oxyhemoglobin saturation at a given arterial oxygen pressure (PaO2) (i.e., 40 mm Hg) is lower in patients with sickle cell disease (SCD) (right dashed line) than would be predicted by a normal oxyhemoglobin dissociation curve (solid line) because of increased 2,3 diphosphoglycerate (2,3 DPG) in sickled erythrocytes, as an adaption to severe anemia to prevent tissue hypoxia, or in the setting of hypoventilation with CO2 retention from pain, opioid use, and/or sleep-disordered breathing. The oxygen pressure (Po2) at which hemoglobin (Hb) is 50% saturated is about 27 mm Hg in normal subjects and about 33 mm Hg in patients with SCD. Reprinted by permission from Reference 178.
Although the gold standard for diagnosis of SDB is overnight, in-laboratory PSG for adults (114) and children (115), it is expensive and burdensome for patients. Unfortunately, there are insufficient data about accuracy of home sleep apnea testing or other modalities in patients with cardiorespiratory comorbidities (116, 117), including SCD. History and physical examination are inadequate for identifying OSA in children (118, 119). Although sleep disruption can be assessed by motion-tracking devices such as actigraphy, accuracy is reduced in the presence of other sleep disorders (120). Relationships between SCD comorbidities and SDB and/or hypoxemia need further investigation, including poor sleep quality, insomnia, nocturnal enuresis, depression, and pain (121–125).
Areas for investigation include: 1) identification of oxygen saturation targets at rest and during exertion, sleep, and acute illness; and 2) evaluation of alternative modalities to improve access to evaluation for SDB (Table 3).
Table 3.
Available modalities for the diagnosis of sleep disorders and nocturnal oxygen desaturation
| Modality | Description | Advantages | Disadvantages |
|---|---|---|---|
| Full polysomnography | Full channel, in-laboratory with audiovisual recording; technician-attended study; measures cardiorespiratory parameters, limb movements, sleep staging, and quality | “Gold standard” physiological measurement of sleep, breathing, and of sleep and breathing; can be used in individuals with complex medical problems | Expensive, burdensome, limited access, often with long wait times |
| Home sleep apnea testing | Option A: Limited channel unattended study in the home that measures cardiorespiratory parameters: pulse oximetry, chest wall movement, nasal air flow; ±snoring, ECG | Home based, less expensive, more convenient; valid method to diagnose OSA in uncomplicated adult patients, but data in pediatric population sparse and no data for SCD | Not recommended for those with chronic conditions including cardiopulmonary comorbidities (117); does not assess sleep quality; may underestimate disease severity; not recommended for children |
| Option B: Devices measuring pulse oximetry, actigraphy and estimating sleep and SDB from arterial tonometry (WatchPAT) | Validated for OSA diagnosis in adults without chronic cardiorespiratory conditions | Not validated in children or SCD populations | |
| History and physical examination | Inquiring about snoring and daytime sleepiness; assessment of tonsil size | Inexpensive, noninvasive | Poor sensitivity and specificity for OSA; individuals may not be aware of snoring; SDB exists in absence of adenotonsillar hypertrophy and may persist A/T |
| Pulse oximetry | Noninvasive measure of oxyhemoglobin saturation | Low cost, easy to use, well tolerated | Results confounded by dyshemoglobins, questionable reliability in severe anemia and illness, limited by motion artifact |
| Pulse CO-oximetry | Noninvasive measure of carboxyhemoglobin and methemoglobin via 8 wavelength spectrophotometry | Estimates contribution of carboxyhemoglobin and methemoglobin to decreased SpO2; agrees with blood CO-oximetry; easy to use, well-tolerated (179) | Not widely used, may be cost prohibitive, limited by motion artifact |
| Actigraphy | Wrist device using accelerometer technology to estimate wake–sleep patterns and sleep disruption | Low cost, noninvasive, well tolerated, can be used in the home | Accuracy varies across devices; results confounded by other sleep disorders (OSA, periodic limb movement); estimates sleep, but not SDB |
Definition of abbreviations: A/T = adenotonsillectomy; ECG = electrocardiogram; OSA = obstructive sleep apnea; SCD = sickle cell disease; SDB = sleep-disordered breathing; SpO2 = oxygen saturation as measured by pulse oximetry; WatchPAT = Watch-peripheral arterial tone.
Consequences of SDB and Recurrent Oxygen Desaturation
Research question: How do OSA, sustained versus intermittent oxygen desaturation, and repeated arousals during sleep impact SCD morbidity and mortality?
Supporting question:
-
•
Which oxygen saturation threshold for nocturnal hypoxemia contributes to SCD morbidity?
Relationships between SCD pathophysiology and oxygen delivery to the vasculature and organs are complex and multifactorial. Studies suggest that oxyhemoglobin desaturation may predispose to VOEs (126) and ACS (28), but findings are inconsistent (89, 127). A recent prospective cohort of children failed to demonstrate an association between OSA and pain or ACS. Nocturnal oxygen desaturation increased stroke risk in one study (128) but not another (129). Oxyhemoglobin desaturation may adversely affect neurocognitive function, verbal IQ (130), and executive function (131); however, these findings need confirmation in larger cohorts. Notably, the clinical and pathophysiologic significance of SDB and chronic and/or recurrent oxygen desaturation has not been studied in adults with SCD. Although the ATS guidelines for PH in SCD recommend evaluating suspected OSA with polysomnography (132), there have been no studies evaluating OSA as a potential risk factor for PH, PH-associated mortality, or other adverse cardiac outcomes in SCD.
Studies evaluating associations between SDB and clinical outcomes need to account for the impacts of desaturation severity and sleep quality. Tissue hypoxia promotes endothelial injury and hypoxic pulmonary vasoconstriction. Nocturnal oxygen desaturation has been associated with biomarkers of hemolysis (133) and endothelial activation and adhesion (134). Associations between poor sleep quality, SDB, and desaturation with depression and pain are likely multidirectional (125, 126, 135–137) and need further investigation.
Treatment of SDB and Recurrent Oxygen Desaturation
Research question: What is the optimal treatment of SDB among individuals with SCD?
Supporting questions:
-
•
What is the acceptability of treatment for hypoxemia and SDB?
-
•
What is the impact of treatment of hypoxemia and SDB on short and long-term outcomes in SCD?
Acceptability and impact of treating hypoxemia and SDB in SCD are important research priorities. One 6-week pilot trial of auto-adjusting continuous positive airway pressure in 24 children with SCD and OSA demonstrated good adherence and improved sleep parameters, oxygen saturation, and reported pain days (138). No studies have compared adherence among treatment modalities in SCD. Small retrospective cohort studies demonstrate improvements in PSG parameters after adenotonsillectomy for OSA (139–141); however, postoperative complications, including ACS, have been reported (142). Three retrospective studies demonstrate improvements in OSA and nocturnal and daytime oxygen saturation after treatment with hydroxyurea (143–145). No studies to date have evaluated the long-term impact of SDB treatment on VOEs, cardiopulmonary and neurocognitive outcomes, or vascular dysfunction.
Pulmonary Vascular Complications of SCD
The pulmonary vascular complications of SCD include PH, pulmonary artery thrombosis, and venous thromboembolism (VTE). PH is a mortality risk factor, occurs in 6% to 11% of adults with SCD (146–148), and reflects a spectrum of hemodynamics, including precapillary PH (40%) and postcapillary PH (60%) (149); mortality risk increases with worsening hemodynamics and right ventricular dysfunction (150, 151).
SCD is a hypercoagulable state (Table 4); as such, patients have a fourfold increase in VTE compared with the general population (152). Thrombosis risk is hard to predict in individual patients. There are no SCD-specific guidelines or clinical trials of anticoagulant therapies.
Table 4.
Risk factors for venous thromboembolism in sickle cell disease
| Traditional VTE Risk Factors | SCD-related VTE Risk Factors |
|---|---|
| Immobilization due to frequent hospitalizations (1) | Coagulation factors |
| Use of central venous catheters for venous access and red cell exchange/transfusion therapy (180) | Decreased protein C, protein S, and antithrombin III levels |
| Surgery | Decreased factor XII levels (181) |
| Orthopedic surgery (avascular necrosis of hip, shoulder) | Elevated circulating antiphospholipid antibodies (182) |
| Cholecystectomy | Elevated plasma levels of thrombin–antithrombin complexes, prothrombin fragment 1 + 2 (a marker of thrombin and fibrin generation and platelet activation) (183) |
| Increased pregnancy-related VTE | During VOE |
| Splenectomy: functional asplenia and postsurgical splenectomy | Abnormal externalization of phosphatidylserine in erythrocytes and adherence to the vascular endothelium |
| Increased tissue factor expression | |
| Increased circulating fibrinogen, vWF, and factors VII and VIII | |
| Impaired fibrinolysis | |
| Upregulation of cellular adhesion molecules |
Definition of abbreviations: SCD = sickle cell disease; VOE = vaso-occlusive events; VTE = venous thromboembolism: vWF = Von Willibrand’s factor.
Priority areas for research in SCD-PH include: 1) understanding the impact of early identification of PH on clinical outcomes, and 2) the best therapeutic strategy for SCD-PH. Priority research areas in patients with VTE in SCD include: 1) management of thromboprophylaxis in pregnant women and children, and 2) approach to anticoagulant therapy for a first-time VTE.
Impact of PH Diagnosis on Outcomes
Research question: Does the early identification and treatment of PH in SCD improve outcomes?
Supporting questions:
-
•
In the asymptomatic patient, does evaluation for SCD-PH improve clinical outcomes?
-
•
What is the best strategy to screen for SCD-PH?
-
•
What should be the approach to abnormal screening studies or the diagnosis of the symptomatic patient?
An elevated TRV by echocardiography (defined as ≥2.5 m/s) occurs in approximately one-third of hemoglobin-SS adults, 10% to 20% of hemoglobin-SC adults, and 10% to 20% of HbSS children and adolescents (8, 132, 153). An elevated TRV on echocardiography can be used to estimate right ventricular or pulmonary artery systolic pressure. Values in adults ≥2.5 m/s identify 25% to 44% of patients with PH assessed by right heart catheterization, and values ≥3.0 m/s identify about 75% of patients with pulmonary hypertension (149). In adults, even borderline values (≥2.5 m/s) are associated with early mortality (8). In contrast, in children and adolescents, an elevated TRV does not increase mortality risk but does predict reduced exercise capacity (18, 132, 154), However, there are no right heart catheterization data in children, which would be needed to better interpret TRV values in this age group. An elevated N-terminal pro-brain natriuretic peptide level or a reduced 6-minute-walk distance increases TRV specificity for PH (124).
An approach to evaluate an elevated TRV in SCD was proposed as part of the ATS Clinical Guidelines for Diagnosis and Treatment of PH in SCD (132). Screening with TRV was proposed to identify patients at high risk of having PH and increased mortality, to intensify hematological therapy with hydroxyurea or chronic transfusion therapy, and also to identify patients with treatable forms of PH, for example group I pulmonary arterial hypertension (PAH) and group IV chronic thromboembolic PH. A recent retrospective study of 13 HbSS patients with precapillary PH reported that chronic transfusion therapy improved New York Heart Association functional class and hemodynamics, particularly pulmonary vascular resistance (P = 0.01) (155). Right heart catheterization is necessary for PH diagnosis and classification, yet inconsistency remains across centers regarding when this is pursued.
Despite the mortality risk and identification of elevated TRV, it is unclear whether interventions in response to abnormal echocardiograms affect clinical outcomes (9, 132). Echocardiography is, however, widely accepted in the evaluation of dyspnea, a commonly reported symptom in adults with SCD (9, 132, 156). Studies demonstrate increased mortality in adults with SCD with PH or an isolated elevated TRV or N-terminal pro-brain natriuretic peptide level (8, 150, 157, 158). Whether increased mortality can be ameliorated with early intervention is unclear, because randomized trials have not been completed.
Two recent guideline documents offer conflicting recommendations (9, 132). The ATS guidelines recommend screening all patients 18 years and older, whereas the NHLBI guidelines for the care of patients with SCD do not. Optimal PH screening tests and frequency of testing have not been determined. Elevated TRV is not associated with mortality among children and adolescents, weakening the argument for screening pediatric age groups (132, 159, 160). However, the association with progressive exercise limitation in children with higher TRV values suggests that studies may be needed to evaluate screening in children for cardiopulmonary complications and then to follow them to adulthood to identify higher-risk groups for intervention. It is unknown whether newer modalities, such as cardiac magnetic resonance imaging, could be more informative than echocardiography (151).
Treatment of SCD-related PAH
Research question: Do patients with SCD-PAH respond to PAH therapy?
Supporting questions:
-
•
Which criteria should be used to determine which patients should receive PAH therapy?
-
•
Which novel PAH therapeutics hold the most promise for patients with SCD-PAH?
-
•
What should be the treatment approach for patients with PH related to left-sided heart disease?
PH in SCD represents a spectrum of hemodynamic and clinical findings; those with precapillary PH similar to PAH are most appropriate to study for efficacy of PAH therapy. The use of U.S. Food and Drug Administration–approved PAH medications for SCD-PAH is controversial, as no randomized trials in SCD have been completed (161, 162). In SCD-PAH, the strongest support for PAH-targeted therapy comes from seven case series, in which 53 patients with SCD and precapillary PH hemodynamics (six with chronic thromboembolic PH) who received targeted PAH therapies had improved 6-minute-walk distance (157, 163–166). Improvements in mean pulmonary arterial pressure, pulmonary vascular resistance, and cardiac index occurred but were only assessed in a subset of patients (164). Three randomized trials of bosentan or sildenafil in SCD were stopped early and were underpowered to address efficacy. Two of the clinical trials evaluating bosentan for pre- and postcapillary PH (ASSET 1 and 2) were terminated by the sponsor because of underenrollment (161). The trial of sildenafil for the treatment of PH in SCD enrolled patients on the basis of an elevated TRV and not by right heart catheterization–proven PAH (162). The sildenafil trial was stopped after enrollment of 72 subjects because of an increased rate of hospitalizations, particularly for vaso-occlusive crises in the sildenafil-treated group. PAH therapy may be most appropriate in those with pre-capillary hemodynamics, similar to Group 1 PAH (132). Despite similarities in hemodynamics, hemoglobinopathy-related complications in these patients emphasize the importance of clinical trials in SCD (167).
Deep Venous Thrombosis Prophylaxis in Specific Cohorts of Patients with SCD
Research question: How should deep venous thrombosis (DVT) prophylaxis be approached in pregnant women and children with SCD?
SCD is a risk factor for pregnancy-related VTE (168–170), yet there are no studies of thromboprophylaxis in this population. Standard of care includes thromboprophylaxis for all adult hospitalized patients with SCD (171), and current guidelines recommend the consideration of outpatient VTE prophylaxis in pregnant women with SCD and prior VTE (152, 172). The high risk of VTE in pregnancy in SCD raises the question of whether thromboprophylaxis is warranted in all pregnant patients.
Evidence regarding thromboprophylaxis in the pediatric SCD population is limited. VTE in pediatric patients with SCD appears to be rare, and cases are often catheter related (173). Thromboprophylaxis is generally reserved for children with additional risk factors for thrombosis in addition to SCD alone (174). There may be increased hemorrhagic risk in patients with SCD receiving anticoagulant therapy because of retinal and cerebral vasculopathy, which raises concern for universal use. The epidemiology of pediatric SCD-VTE needs further investigation.
Secondary Prevention of DVT, Pulmonary Embolism, or Pulmonary Artery Thrombosis
Research question: If a patient with SCD is diagnosed with a DVT, pulmonary embolism, or pulmonary artery thrombosis, what treatment should be used and for what duration?
Supporting question:
-
•
What are indications for long-term anticoagulation in SCD?
Patients with SCD with an isolated, first-time DVT or pulmonary embolism currently receive standard American College of Chest Physicians guideline–directed anticoagulation for 3 to 6 months (175). A retrospective study using patient discharge data reported a 31.3% VTE recurrence risk at 5 years, which suggests that this approach may be inadequate (176). For patients with SCD-PH and VTE, consensus guidelines recommend indefinite anticoagulation for those without significant hemorrhagic risk (132). The rate of recurrence of VTE and the impact of PE on cardiopulmonary function should be studied to better understand how different treatment regimens impact clinical outcomes.
FUTURE DIRECTIONS
When assessing the most important questions facing the field of sickle cell lung disease, we must keep an eye toward the future. Curative therapies, including hematopoietic stem-cell transplantation and gene therapy, are currently under investigation in SCD. Although pulmonary comorbidities, including an elevated TRV, are among the inclusion criteria for many of these studies, we do not know whether, or how, the course of pulmonary disease is altered by “curing” the hemoglobinopathy.
It is critical that adult and pediatric pulmonologists take an active role in the design and analyses of these studies. In addition, we must focus on training the future generation of pediatric and, particularly, adult pulmonologists to develop a cadre of experts who can work together to move the field of sickle cell lung disease forward.
Given that diseases affecting underserved populations frequently receive less research funding (177), it will take a concerted effort to develop high-quality clinical research studies of sickle cell lung disease. We advocate for targeted funding initiatives from the U.S. National Institutes of Health and specialty organizations, such as the ATS, the American Society of Hematology, the European Respiratory Society, and the American Society of Human Genetics, for international, multicenter, longitudinal studies that include high-prevalence and low-resource settings as well as training grants targeted toward clinical investigators to promote these efforts in a systematic fashion.
SUMMARY
Important questions relating to sickle cell lung disease remain unanswered. There are multiple barriers to characterizing sickle cell lung disease, including a lack of multicenter prospective studies, inadequate research funding, and inconsistent phenotyping strategies. Patient registries are critical to study the natural history of this disease. High-quality, multicenter, longitudinal cohort studies and randomized clinical trials designed and implemented by multidisciplinary teams are the best way to evaluate the questions outlined in this report and advance the care of patients with pulmonary complications of SCD.
Supplementary Material
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Pediatrics.
Members of the subcommittee are as follows:
A. Parker Ruhl, M.D., M.H.S.1,2 (Co-Chair)
S. Christy Sadreameli, M.D., M.H.S.3 (Co-Chair)
Robyn T. Cohen, M.D., M.P.H.4 (Co-Chair)
Elizabeth S. Klings, M.D.5 (Co-Chair)
Julian L. Allen, M.D.6,7
Debra P. Bennett, B.A.8
Andrew D. Campbell, M.D.9
Thomas D. Coates, M.D.10
Dapa A. Diallo, M.D.11
Joshua J. Field, M.D.12
Elizabeth K. Fiorino, M.D.13
Mark T. Gladwin, M.D.14,15
Jeffrey A. Glassberg, M.D., M.H.S.16
Victor R. Gordeuk, M.D.17
Leroy M. Graham, M.D.18
Anne Greenough, M.D.19
Jo Howard, M.B.B.Chir.10,20
Gregory J. Kato, M.D.14,15,21
Jennifer Knight-Madden, M.B.B.S.22
Benjamin T. Kopp, M.D., M.P.H.23,24
Anastassios C. Koumbourlis, M.D.25
Sophie M. Lanzkron, M.D.26
Robert I. Liem, M.D., M.S.27
Roberto F. Machado, M.D.28
Alem Mehari, M.D.29
Claudia R. Morris, M.D.30
Folasade O. Ogunlesi, M.D.25
Carol L. Rosen, M.D.31
Kim Smith-Whitley, M.D.32
Danna Tauber, M.D.6,7
Nancy Terry, M.S.33
Swee Lay Thein, M.B.B.S., D.Sc.34
Elliott Vichinsky, M.D.35
Nargues A. Weir, M.D.2
1Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; 2Pulmonary Branch and 34Sickle Cell Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland; 3Eudowood Division of Pediatric Respiratory Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland; 4Division of Pediatric Pulmonary and Allergy, Department of Pediatrics, and 5The Pulmonary Center, Boston University School of Medicine, Boston, Massachusetts; 6Division of Pulmonary Medicine, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; 7Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; 8Patient Advocate, Baltimore, Maryland; 9Center for Cancer and Blood Disorders, Children’s National Medical Center, Washington, District of Columbia; 10Division of Hematology/Oncology, Children’s Hospital Los Angeles, Los Angeles, California; 11Centre de Recherche et de Lutte contre la Drépanocytose, University of Sciences, Techniques and Technologies of Bamako, Bamako, Mali; 12Medical Sciences Institute, Blood Center of Wisconsin, Department of Medicine, Medical College of Wisconsin, Milwaukee, Wisconsin; 13Division of Pulmonary Medicine, Cohen Children’s Medical Center, Department of Pediatrics, Zucker School of Medicine at Hofstra-Northwell, Hempstead, New York; 14Division of Pulmonary, Allergy, and Critical Care Medicine, 15Heart, Lung, and Blood Vascular Medicine Institute, and 21Division of Hematology-Oncology, Department of Medicine, University of Pittsburgh Medical Center, Pennsylvania; 16Departments of Emergency Medicine, Hematology and Medical Oncology, Icahn School of Medicine at Mount Sinai, New York, New York; 17Division of Hematology-Oncology, University of Illinois at Chicago, Chicago, Illinois; 18Bridge Atlanta Medical Center, Atlanta, Georgia; 19Asthma UK Centre in Allergic Mechanisms of Asthma and Department of Women and Children’s Health, School of Life Course Sciences, King’s College London, London, United Kingdom; 20Department of Haematology, Guy’s and St. Thomas’ Hospital, London, United Kingdom; 22The Sickle Cell Unit, Caribbean Institute for Health Research, the University of the West Indies, Kingston, Jamaica; 23Department of Pediatrics, The Ohio State University College of Medicine, Columbus, Ohio; 24Nationwide Children’s Hospital, Columbus, Ohio; 25Division of Pulmonary and Sleep Medicine, Children’s National Health System, George Washington University, School of Medicine and Health Sciences, Washington, District of Columbia; 26Division of Hematology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland; 27Division of Hematology, Oncology, and Stem Cell Transplant, Ann and Robert H. Lurie Children’s Hospital of Chicago, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, Illinois; 28Division of Pulmonary, Critical Care, Sleep, and Occupational Medicine, Indiana University School of Medicine, Indianapolis, Indiana; 29Division of Pulmonary Diseases, Howard University College of Medicine, Washington, District of Columbia; 30Division of Pediatric Emergency Medicine, Department of Pediatrics, and the Emory Children’s Center for Cystic Fibrosis and Airways Disease Research, Emory University School of Medicine, Atlanta, Georgia; 31University Hospitals of Cleveland, Rainbow Babies and Children’s Hospital, Case Western Reserve University School of Medicine, Cleveland, Ohio; 32Division of Hematology, The Children’s Hospital of Philadelphia, Perelman School of Medicine of the University of Pennsylvania, Philadelphia, Pennsylvania; 33National Institutes of Health Library, Bethesda, Maryland; and 35Department of Hematology/Oncology, Children's Hospital Oakland Research Institute, Oakland, California.
Acknowledgment
The committee thanks the American Thoracic Society for funding this document and the ATS staff for administrative assistance with conference calls and face-to-face meetings; and the clinicians and sickle cell disease patients who have participated in the studies that have made this document possible.
Footnotes
This Official Workshop Report of the American Thoracic Society was approved May 2019
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1513/AnnalsATS.201906-433ST.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health, the National Health Service of the United Kingdom, the National Institute of Health Research of the United Kingdom, or the Department of Health of the United Kingdom.
Author disclosures: A.D.C. received research support for Global Therapeutics; served as a speaker for Global Therapeutics and Ironwood Pharmaceuticals. T.D.C. served on an advisory committee for Apopharma, Agios, Bio Products Laboratory, Celgene, Novartis and Vifor; served on a data and safety monitoring board for Sangamo. J.J.F. received research support from Astellas, Incyte, Ironwood, NKTT, and Prolong; served as a consultant for Ironwood; served on a data and safety monitoring board for Bayer. E.K.F. served as a consultant for Audentes Therapeutics. M.T.G. received research support and served on an advisory committee for Bayer; served as a consultant for Acceleron, Actelion, Catalyst Biosciences, Epizyme, Modus Therapeutics, United Therapeutics and Sujana Biotech; serves as a shareholder, advisor, and director in Globin Solutions, Inc.; co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which were previously licensed to United Therapeutics and Hope Pharmaceuticals, and is now licensed to Globin Solutions; co-inventor of pending patent applications and planned patents directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning, which have recently been licensed by Globin Solutions, Inc. J.A.G. received research support from Pfizer. V.R.G. served as a consultant for Emmaus, Global Blood Therapeutics, Modus Therapeutics and Novartis. L.M.G. received personal fees from Boehringer Ingelheim, Novartis and ThermoFisher. A.G. received research support from and served as a speaker for Abbott Laboratories, MedImmune and SLE. J.H. served on an advisory committee for Bluebird Bio and Global Blood Therapeutics; served as a speaker for Add Medica, Novartis and Terumo BCT; received travel, lodging, meals, or entertainment from Imara and Resonance Health; served as a consultant for Imara; received personal fees from Aes Rx. G.J.K. received research support from Bayer; served on an advisory committee for Mast Therapeutics; served as a consultant for Bioverativ, Global Blood Therapeutics and Novartis. B.T.K. served on an advisory committee for Vertex. S.M.L. received research support from Ironwood Pharmaceuticals, Global Blood Therapeutics, Pfizer, Prolong and Selexys. R.I.L. received research support from Global Blood Therapeutics. C.R.M. served as a consultant for Pfizer; received royalties, licensing fees, or other sales proceeds from Lifetriene. C.L.R. received research support from Avadel Pharmaceuticals and Jazz Pharmaceuticals; served as a consultant from Jazz Pharmaceuticals. K.S.-W. served on an advisory committee for Bioverativ, Celegen, Cerus, Global Blood Therapeutics, Novartis and Pfizer. E.S.K. received research support from Actelion, Arena/United Therapeutics, Bayer, Eiger, Incyte, Reata Pharmaceuticals; served as a consultant for Pfizer; received royalties from UpToDate; served on a data and safety monitoring board for Micelle. A.P.R., S.C.S., J.L.A., D.P.B., D.A.D., J.K.-M., A.C.K., R.F.M., A.M., F.O.O., D.T., N.T., S.L.T., E.V., N.A.W., and R.T.C. reported no relevant commercial relationships.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Pediatrics
References
- 1.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi S, Ghafuri DL, Glassberg J, Kassim AA, Rodeghier M, DeBaun MR. Rapidly progressive acute chest syndrome in individuals with sickle cell anemia: a distinct acute chest syndrome phenotype. Am J Hematol. 2016;91:1185–1190. doi: 10.1002/ajh.24539. [DOI] [PubMed] [Google Scholar]
- 3.Mehari A, Klings ES. Chronic pulmonary complications of sickle cell disease. Chest. 2016;149:1313–1324. doi: 10.1016/j.chest.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 5.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 6.Kassim AA, Payne AB, Rodeghier M, Macklin EA, Strunk RC, DeBaun MR. Low forced expiratory volume is associated with earlier death in sickle cell anemia. Blood. 2015;126:1544–1550. doi: 10.1182/blood-2015-05-644435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatr Pulmonol. 2008;43:1061–1066. doi: 10.1002/ppul.20883. [DOI] [PubMed] [Google Scholar]
- 8.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 9.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 10.Fitzhugh CD, Lauder N, Jonassaint JC, Telen MJ, Zhao X, Wright EC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85:36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston M, Smith J, Gallagher D, Flournoy-Gill Z, West S, Bellevue R, et al. Recruitment in the Cooperative Study of Sickle Cell Disease (CSSCD) Control Clin Trials. 1987;8:131S–140S. doi: 10.1016/0197-2456(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez O, Yovetich NA, Scott JP, Owen W, Miller ST, Schultz W, et al. Investigators of the Stroke With Transfusions Changing to Hydroxyurea Clinical Trial (SWiTCH) Pain and other non-neurological adverse events in children with sickle cell anemia and previous stroke who received hydroxyurea and phlebotomy or chronic transfusions and chelation: results from the SWiTCH clinical trial. Am J Hematol. 2013;88:932–938. doi: 10.1002/ajh.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen RT, Strunk RC, Rodeghier M, Rosen CL, Kirkham FJ, Kirkby J, et al. Pattern of lung function is not associated with prior or future morbidity in children with sickle cell anemia. Ann Am Thorac Soc. 2016;13:1314–1323. doi: 10.1513/AnnalsATS.201510-706OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arteta M, Campbell A, Nouraie M, Rana S, Onyekwere OC, Ensing G, et al. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36:185–189. doi: 10.1097/MPH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field JJ, Glassberg J, Gilmore A, Howard J, Patankar S, Yan Y, et al. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83:574–576. doi: 10.1002/ajh.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–782. doi: 10.1542/peds.2007-0730. [DOI] [PubMed] [Google Scholar]
- 18.Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–347. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrita IZ, Mohammed A, Layton M, Ghorashian S, Gilmore A, Cho G, et al. The association between tricuspid regurgitation velocity and 5-year survival in a North West London population of patients with sickle cell disease in the United Kingdom. Br J Haematol. 2013;162:400–408. doi: 10.1111/bjh.12391. [DOI] [PubMed] [Google Scholar]
- 20.Maitra P, Caughey M, Robinson L, Desai PC, Jones S, Nouraie M, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102:626–636. doi: 10.3324/haematol.2016.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, et al. Investigators, Comprehensive Sickle Cell Centers. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85:6–13. doi: 10.1002/ajh.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charache S, Scott JC, Charache P. “Acute chest syndrome” in adults with sickle cell anemia: microbiology, treatment, and prevention. Arch Intern Med. 1979;139:67–69. [PubMed] [Google Scholar]
- 24.Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, et al. National Acute Chest Syndrome Study Group. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 25.Lazarus SG, Kelleman M, Adisa O, Zmitrovich AR, Hagbom R, Cohen S, et al. Are we missing the mark? Fever, respiratory symptoms, chest radiographs, and acute chest syndrome in sickle cell disease. Am J Hematol. 2016;91:E332–E333. doi: 10.1002/ajh.24408. [DOI] [PubMed] [Google Scholar]
- 26.Miller AC, Gladwin MT. Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med. 2012;185:1154–1165. doi: 10.1164/rccm.201111-2082CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellet PS, Kalinyak KA, Shukla R, Gelfand MJ, Rucknagel DL. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med. 1995;333:699–703. doi: 10.1056/NEJM199509143331104. [DOI] [PubMed] [Google Scholar]
- 28.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81:3422–3427. [PubMed] [Google Scholar]
- 29.Mekontso Dessap A, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, et al. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177:646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 30.DeBaun MR, Strunk RC. The intersection between asthma and acute chest syndrome in children with sickle-cell anaemia. Lancet. 2016;387:2545–2553. doi: 10.1016/S0140-6736(16)00145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resende Cardoso PS, Lopes Pessoa de Aguiar RA, Viana MB. Clinical complications in pregnant women with sickle cell disease: prospective study of factors predicting maternal death or near miss. Rev Bras Hematol Hemoter. 2014;36:256–263. doi: 10.1016/j.bjhh.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Styles L, Wager CG, Labotka RJ, Smith-Whitley K, Thompson AA, Lane PA, et al. Sickle Cell Disease Clinical Research Network (SCDCRN) Refining the value of secretory phospholipase A2 as a predictor of acute chest syndrome in sickle cell disease: results of a feasibility study (PROACTIVE) Br J Haematol. 2012;157:627–636. doi: 10.1111/j.1365-2141.2012.09105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapping-Carr G, Khalyfa A, Rangel S, Darlington W, Beyer EC, Peddinti R, et al. Exosomes contribute to endothelial integrity and acute chest syndrome risk: preliminary findings. Pediatr Pulmonol. 2017;52:1478–1485. doi: 10.1002/ppul.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bargoma EM, Mitsuyoshi JK, Larkin SK, Styles LA, Kuypers FA, Test ST. Serum C-reactive protein parallels secretory phospholipase A2 in sickle cell disease patients with vasoocclusive crisis or acute chest syndrome. Blood. 2005;105:3384–3385. doi: 10.1182/blood-2004-12-4676. [DOI] [PubMed] [Google Scholar]
- 35.Michael DD. sPLA2 in EBC during acute chest syndrome. ClinicalTrialsgov, Identifier NCT03250585; 2017.
- 36.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 37.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, et al. DeNOVO Investigators. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neocleous C, Spanou C, Adramerina A, Spyrou G, Tzanetis F. Painful vaso-occlusive crisis as a prodromal phase of acute chest syndrome: is only one chest X-ray enough? A case report. Prague Med Rep. 2013;114:180–185. doi: 10.14712/23362936.2014.21. [DOI] [PubMed] [Google Scholar]
- 39.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 40.Niihara Y, Miller ST, Kanter J, Lanzkron S, Smith WR, Hsu LL, et al. Investigators of the Phase 3 Trial of l-Glutamine in Sickle Cell Disease. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379:226–235. doi: 10.1056/NEJMoa1715971. [DOI] [PubMed] [Google Scholar]
- 41.Strouse JJ, Takemoto CM, Keefer JR, Kato GJ, Casella JF. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2008;50:1006–1012. doi: 10.1002/pbc.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darbari DS, Fasano RS, Minniti CP, Castro OO, Gordeuk VR, Taylor JG, VI, et al. Severe vaso-occlusive episodes associated with use of systemic corticosteroids in patients with sickle cell disease. J Natl Med Assoc. 2008;100:948–951. doi: 10.1016/S0027-9684(15)31410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin TC, McIntire D, Buchanan GR. High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N Engl J Med. 1994;330:733–737. doi: 10.1056/NEJM199403173301101. [DOI] [PubMed] [Google Scholar]
- 44.Bernini JC, Rogers ZR, Sandler ES, Reisch JS, Quinn CT, Buchanan GR. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–3089. [PubMed] [Google Scholar]
- 45.Quinn CT, Stuart MJ, Kesler K, Ataga KI, Wang WC, Styles L, et al. Investigators of the Comprehensive Sickle Cell C. Tapered oral dexamethasone for the acute chest syndrome of sickle cell disease. Br J Haematol. 2011;155:263–267. doi: 10.1111/j.1365-2141.2011.08827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glassberg J, Minnitti C, Cromwell C, Cytryn L, Kraus T, Skloot GS, et al. Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: a triple-blind, randomized trial. Am J Hematol. 2017;92:622–631. doi: 10.1002/ajh.24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langer AL, Leader A, Kim-Schulze S, Ginzburg Y, Merad M, Glassberg J. Inhaled steroids associated with decreased macrophage markers in nonasthmatic individuals with sickle cell disease in a randomized trial. Ann Hematol. 2019;98:841–849. doi: 10.1007/s00277-019-03635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Telen MJ. Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease. Blood. 2016;127:810–819. doi: 10.1182/blood-2015-09-618553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nansseu JR, Alima Yanda AN, Chelo D, Tatah SA, Mbassi Awa HD, Seungue J, et al. The acute chest syndrome in Cameroonian children living with sickle cell disease. BMC Pediatr. 2015;15:131. doi: 10.1186/s12887-015-0454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asare EV, Olayemi E, Boafor T, Dei-Adomakoh Y, Mensah E, Osei-Bonsu Y, et al. A case series describing causes of death in pregnant women with sickle cell disease in a low-resource setting. Am J Hematol. 2018;93:E167–E170. doi: 10.1002/ajh.25115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asare EV, Olayemi E, Boafor T, Dei-Adomakoh Y, Mensah E, Ghansah H, et al. Implementation of multidisciplinary care reduces maternal mortality in women with sickle cell disease living in low-resource setting. Am J Hematol. 2017;92:872–878. doi: 10.1002/ajh.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 expert panel report. Am Fam Physician. 2015;92:1069–1076. [PubMed] [Google Scholar]
- 54.Koumbourlis AC, Hurlet-Jensen A, Bye MR. Lung function in infants with sickle cell disease. Pediatr Pulmonol. 1997;24:277–281. doi: 10.1002/(sici)1099-0496(199710)24:4<277::aid-ppul6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 55.Catanzaro T, Koumbourlis AC. Somatic growth and lung function in sickle cell disease. Paediatr Respir Rev. 2014;15:28–32. doi: 10.1016/j.prrv.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Boyd JH, DeBaun MR, Morgan WJ, Mao J, Strunk RC. Lower airway obstruction is associated with increased morbidity in children with sickle cell disease. Pediatr Pulmonol. 2009;44:290–296. doi: 10.1002/ppul.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koumbourlis AC, Lee DJ, Lee A. Lung function and somatic growth in patients with hemoglobin SC sickle cell disease. Pediatr Pulmonol. 2008;43:175–178. doi: 10.1002/ppul.20752. [DOI] [PubMed] [Google Scholar]
- 58.Koumbourlis AC, Lee DJ, Lee A. Changes in lung function in children with sickle cell disease. Am J Respir Crit Care Med. 2009;180:377, author reply 377–378. doi: 10.1164/ajrccm.180.4.377. [DOI] [PubMed] [Google Scholar]
- 59.Lunt A, Desai SR, Wells AU, Hansell DM, Mushemi S, Melikian N, et al. Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69:746–751. doi: 10.1136/thoraxjnl-2013-204809. [DOI] [PubMed] [Google Scholar]
- 60.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Hai. 2010;188:499–504. doi: 10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 61.Koumbourlis AC. Lung function in sickle cell disease. Paediatr Respir Rev. 2014;15:33–37. doi: 10.1016/j.prrv.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 62.MacLean JE, Atenafu E, Kirby-Allen M, MacLusky IB, Stephens D, Grasemann H, et al. Longitudinal decline in lung volume in a population of children with sickle cell disease. Am J Respir Crit Care Med. 2008;178:1055–1059. doi: 10.1164/rccm.200708-1219OC. [DOI] [PubMed] [Google Scholar]
- 63.Lunt A, McGhee E, Sylvester K, Rafferty G, Dick M, Rees D, et al. Longitudinal assessment of lung function in children with sickle cell disease. Pediatr Pulmonol. 2016;51:717–723. doi: 10.1002/ppul.23367. [DOI] [PubMed] [Google Scholar]
- 64.Willen SM, Cohen R, Rodeghier M, Kirkham F, Redline SS, Rosen C, et al. Age is a predictor of a small decrease in lung function in children with sickle cell anemia. Am J Hematol. 2018;93:408–415. doi: 10.1002/ajh.25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaturvedi S, Ghafuri DL, Kassim A, Rodeghier M, DeBaun MR. Elevated tricuspid regurgitant jet velocity, reduced forced expiratory volume in 1 second, and mortality in adults with sickle cell disease. Am J Hematol. 2017;92:125–130. doi: 10.1002/ajh.24598. [DOI] [PubMed] [Google Scholar]
- 66.Lunt A, Mortimer L, Rees D, Height S, Thein SL, Greenough A. Heterogeneity of respiratory disease in children and young adults with sickle cell disease. Thorax. 2018;73:575–577. doi: 10.1136/thoraxjnl-2017-210206. [DOI] [PubMed] [Google Scholar]
- 67.DeBaun MR, Rodeghier M, Cohen R, Kirkham FJ, Rosen CL, Roberts I, et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am J Hematol. 2014;89:E212–E217. doi: 10.1002/ajh.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen RT, Madadi A, Blinder MA, DeBaun MR, Strunk RC, Field JJ. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. Am J Hematol. 2011;86:756–761. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glassberg JA, Chow A, Wisnivesky J, Hoffman R, Debaun MR, Richardson LD. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012;159:472–479. doi: 10.1111/bjh.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strunk RC, Cohen RT, Cooper BP, Rodeghier M, Kirkham FJ, Warner JO, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia J Pediatr 2014164821–826.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Field JJ, Stocks J, Kirkham FJ, Rosen CL, Dietzen DJ, Semon T, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139:563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ozbek OY, Malbora B, Sen N, Yazici AC, Ozyurek E, Ozbek N. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42:1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 73.Sen N, Kozanoglu I, Karatasli M, Ermis H, Boga C, Eyuboglu FO. Pulmonary function and airway hyperresponsiveness in adults with sickle cell disease. Hai. 2009;187:195–200. doi: 10.1007/s00408-009-9141-y. [DOI] [PubMed] [Google Scholar]
- 74.Knight-Madden J, Greenough A. Acute pulmonary complications of sickle cell disease. Paediatr Respir Rev. 2014;15:13–16. doi: 10.1016/j.prrv.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol. 2010;30:2392–2399. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Field JJ, Burdick MD, DeBaun MR, Strieter BA, Liu L, Mehrad B, et al. The role of fibrocytes in sickle cell lung disease. PLoS One. 2012;7:e33702. doi: 10.1371/journal.pone.0033702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehrad B, Burdick MD, Wandersee NJ, Shahir KS, Zhang L, Simpson PM, et al. Circulating fibrocytes as biomarkers of impaired lung function in adults with sickle cell disease. Blood Adv. 2017;1:2217–2224. doi: 10.1182/bloodadvances.2017010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonsalves CS, Li C, Mpollo MS, Pullarkat V, Malik P, Tahara SM, et al. Erythropoietin-mediated expression of placenta growth factor is regulated via activation of hypoxia-inducible factor-1α and post-transcriptionally by miR-214 in sickle cell disease. Biochem J. 2015;468:409–423. doi: 10.1042/BJ20141138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross JG, Bernaudin F, Strunk RC, Kamdem A, Arnaud C, Hervé M, et al. Asthma is a distinct comorbid condition in children with sickle cell anemia with elevated total and allergen-specific IgE levels. J Pediatr Hematol Oncol. 2011;33:e205–e208. doi: 10.1097/MPH.0b013e31820db7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127:1440–1446. doi: 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andemariam B, Adami AJ, Singh A, McNamara JT, Secor ER, Guernsey LA, et al. The sickle cell mouse lung: proinflammatory and primed for allergic inflammation. Transl Res. 2015;166:254–268. doi: 10.1016/j.trsl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nandedkar SD, Feroah TR, Hutchins W, Weihrauch D, Konduri KS, Wang J, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008;112:2529–2538. doi: 10.1182/blood-2008-01-132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102:1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 84.Eiymo Mwa Mpollo MS, Brandt EB, Shanmukhappa SK, Arumugam PI, Tiwari S, Loberg A, et al. Placenta growth factor augments airway hyperresponsiveness via leukotrienes and IL-13. J Clin Invest. 2016;126:571–584. doi: 10.1172/JCI77250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lunt A, McGhee E, Robinson P, Rees D, Height S, Greenough A. Lung function, transfusion, pulmonary capillary blood volume and sickle cell disease. Respir Physiol Neurobiol. 2016;222:6–10. doi: 10.1016/j.resp.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Wedderburn CJ, Rees D, Height S, Dick M, Rafferty GF, Lunt A, et al. Airways obstruction and pulmonary capillary blood volume in children with sickle cell disease. Pediatr Pulmonol. 2014;49:716–722. doi: 10.1002/ppul.22845. [DOI] [PubMed] [Google Scholar]
- 87.Bernaudin F, Strunk RC, Kamdem A, Arnaud C, An P, Torres M, et al. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: a retrospective cohort study. Haematologica. 2008;93:1917–1918. doi: 10.3324/haematol.13090. [DOI] [PubMed] [Google Scholar]
- 88.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92:1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 89.Willen SM, Rodeghier M, Strunk RC, Rosen CL, Kirkham FJ, Field JJ, et al. Airway hyperresponsiveness does not predict morbidity in children with sickle cell anemia. Am J Respir Crit Care Med. 2017;195:1533–1534. doi: 10.1164/rccm.201610-1970LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mekontso Dessap A, Contou D, Dandine-Roulland C, Hemery F, Habibi A, Charles-Nelson A, et al. Environmental influences on daily emergency admissions in sickle-cell disease patients. Medicine (Baltimore) 2014;93:e280. doi: 10.1097/MD.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piel FB, Tewari S, Brousse V, Analitis A, Font A, Menzel S, et al. Associations between environmental factors and hospital admissions for sickle cell disease. Haematologica. 2017;102:666–675. doi: 10.3324/haematol.2016.154245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen RT, DeBaun MR, Blinder MA, Strunk RC, Field JJ. Smoking is associated with an increased risk of acute chest syndrome and pain among adults with sickle cell disease. Blood. 2010;115:3852–3854. doi: 10.1182/blood-2010-01-265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadreameli SC, Eakin MN, Robinson KT, Alade RO, Strouse JJ. Secondhand smoke is associated with more frequent hospitalizations in children with sickle cell disease. Am J Hematol. 2016;91:313–317. doi: 10.1002/ajh.24281. [DOI] [PubMed] [Google Scholar]
- 94.Glassberg JA, Wang J, Cohen R, Richardson LD, DeBaun MR. Risk factors for increased ED utilization in a multinational cohort of children with sickle cell disease. Acad Emerg Med. 2012;19:664–672. doi: 10.1111/j.1553-2712.2012.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.West DC, Romano PS, Azari R, Rudominer A, Holman M, Sandhu S. Impact of environmental tobacco smoke on children with sickle cell disease. Arch Pediatr Adolesc Med. 2003;157:1197–1201. doi: 10.1001/archpedi.157.12.1197. [DOI] [PubMed] [Google Scholar]
- 96.Cohen RT, Strunk RC, Field JJ, Rosen CL, Kirkham FJ, Redline S, et al. Environmental tobacco smoke and airway obstruction in children with sickle cell anemia. Chest. 2013;144:1323–1329. doi: 10.1378/chest.12-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLaren A, Klingel M, Behera S, Odame I, Kirby-Allen M, Grasemann H. Effect of hydroxyurea therapy on pulmonary function in children with sickle cell anemia. Am J Respir Crit Care Med. 2017;195:689–691. doi: 10.1164/rccm.201606-1119LE. [DOI] [PubMed] [Google Scholar]
- 98.Glassberg J, Minnitti C, Cromwell C, Cytryn L, Kraus T, Skloot GS, et al. Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: a triple-blind, randomized trial. Am J Hematol. 2017;92:622–631. doi: 10.1002/ajh.24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Hemoglobinopathies and sleep: the road less traveled. Sleep Med Rev. 2015;24:57–70. doi: 10.1016/j.smrv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273–1285. doi: 10.1213/ANE.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 101.Worsham CM, Martin ST, Nouraie SM, Cohen RT, Klings ES. Clinical and laboratory findings associated with sleep disordered breathing in sickle cell disease. Am J Hematol. 2017;92:E649–E651. doi: 10.1002/ajh.24892. [DOI] [PubMed] [Google Scholar]
- 102.Rosen CL, Debaun MR, Strunk RC, Redline S, Seicean S, Craven DI, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134:273–281. doi: 10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pashankar FD, Manwani D, Lee MT, Green NS. Hydroxyurea improves oxygen saturation in children with sickle cell disease. J Pediatr Hematol Oncol. 2015;37:242–243. doi: 10.1097/MPH.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nahavandi M, Tavakkoli F, Wyche MQ, Perlin E, Winter WP, Castro O. Nitric oxide and cyclic GMP levels in sickle cell patients receiving hydroxyurea. Br J Haematol. 2002;119:855–857. doi: 10.1046/j.1365-2141.2002.03919.x. [DOI] [PubMed] [Google Scholar]
- 105.Needleman JP, Franco ME, Varlotta L, Reber-Brodecki D, Bauer N, Dampier C, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 106.Ortiz FO, Aldrich TK, Nagel RL, Benjamin LJ. Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med. 1999;159:447–451. doi: 10.1164/ajrccm.159.2.9806108. [DOI] [PubMed] [Google Scholar]
- 107.Blaisdell CJ, Goodman S, Clark K, Casella JF, Loughlin GM. Pulse oximetry is a poor predictor of hypoxemia in stable children with sickle cell disease. Arch Pediatr Adolesc Med. 2000;154:900–903. doi: 10.1001/archpedi.154.9.900. [DOI] [PubMed] [Google Scholar]
- 108.Eaton WA, Bunn HF. Treating sickle cell disease by targeting HbS polymerization. Blood. 2017;129:2719–2726. doi: 10.1182/blood-2017-02-765891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Needleman JP, Setty BN, Varlotta L, Dampier C, Allen JL. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:423–428. doi: 10.1002/(sici)1099-0496(199912)28:6<423::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 110.Campbell A, Minniti CP, Nouraie M, Arteta M, Rana S, Onyekwere O, et al. Prospective evaluation of haemoglobin oxygen saturation at rest and after exercise in paediatric sickle cell disease patients. Br J Haematol. 2009;147:352–359. doi: 10.1111/j.1365-2141.2009.07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desai PC, Kendel N, Huang Y, Heinlein M, Kraut E, Raman S. Hypoxia in sickle cell disease due to right to left shunting. Am J Hematol. 2019;94:E53–E55. doi: 10.1002/ajh.25360. [DOI] [PubMed] [Google Scholar]
- 112.Dowling MM, Quinn CT, Ramaciotti C, Kanter J, Osunkwo I, Inusa B, et al. PFAST Investigators. Increased prevalence of potential right-to-left shunting in children with sickle cell anaemia and stroke. Br J Haematol. 2017;176:300–308. doi: 10.1111/bjh.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nouraie M, Lee JS, Zhang Y, Kanias T, Zhao X, Xiong Z, et al. Walk-PHASST Investigators and Patients. The relationship between the severity of hemolysis, clinical manifestations and risk of death in 415 patients with sickle cell anemia in the US and Europe. Haematologica. 2013;98:464–472. doi: 10.3324/haematol.2012.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 115.Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, et al. American Academy of Sleep Medicine. Practice parameters for the respiratory indications for polysomnography in children. Sleep (Basel) 2011;34:379–388. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kirk V, Baughn J, D’Andrea L, Friedman N, Galion A, Garetz S, et al. American Academy of Sleep Medicine position paper for the use of a home sleep apnea test for the diagnosis of OSA in children. J Clin Sleep Med. 2017;13:1199–1203. doi: 10.5664/jcsm.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 119.Kang KT, Weng WC, Lee CH, Hsiao TY, Lee PL, Lee YL, et al. Detection of pediatric obstructive sleep apnea syndrome: history or anatomical findings? Sleep Med. 2015;16:617–624. doi: 10.1016/j.sleep.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 120.Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep. 2007;30:899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogers VE, Marcus CL, Jawad AF, Smith-Whitley K, Ohene-Frempong K, Bowdre C, et al. Periodic limb movements and disrupted sleep in children with sickle cell disease. Sleep (Basel) 2011;34:899–908. doi: 10.5665/SLEEP.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Souza LC, Viegas CA. Quality of sleep and pulmonary function in clinically stable adolescents with sickle cell anemia. J Bras Pneumol. 2007;33:275–281. doi: 10.1590/s1806-37132007000300008. [DOI] [PubMed] [Google Scholar]
- 123.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6:374–381. [PMC free article] [PubMed] [Google Scholar]
- 124.Daniel LC, Grant M, Kothare SV, Dampier C, Barakat LP. Sleep patterns in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55:501–507. doi: 10.1002/pbc.22564. [DOI] [PubMed] [Google Scholar]
- 125.Wallen GR, Minniti CP, Krumlauf M, Eckes E, Allen D, Oguhebe A, et al. Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry. 2014;14:207. doi: 10.1186/1471-244X-14-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 127.Willen SM, Rodeghier M, Rosen CL, DeBaun MR. Sleep disordered breathing does not predict acute severe pain episodes in children with sickle cell anemia. Am J Hematol. 2018;93:478–485. doi: 10.1002/ajh.25013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 129.Goldstein NA, Keller R, Rey K, Rao S, Weedon J, Dastgir G, et al. Sleep-disordered breathing and transcranial Dopplers in sickle cell disease. Arch Otolaryngol Head Neck Surg. 2011;137:1263–1268. doi: 10.1001/archoto.2011.190. [DOI] [PubMed] [Google Scholar]
- 130.Hill CM, Hogan AM, Onugha N, Harrison D, Cooper S, McGrigor VJ, et al. Increased cerebral blood flow velocity in children with mild sleep-disordered breathing: a possible association with abnormal neuropsychological function. Pediatrics. 2006;118:e1100–e1108. doi: 10.1542/peds.2006-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hollocks MJ, Kok TB, Kirkham FJ, Gavlak J, Inusa BP, DeBaun MR, et al. Nocturnal oxygen desaturation and disordered sleep as a potential factor in executive dysfunction in sickle cell anemia. J Int Neuropsychol Soc. 2012;18:168–173. doi: 10.1017/S1355617711001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klings ES, Machado RF, Barst RJ, Morris CR, Mubarak KK, Gordeuk VR, et al. American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189:727–740. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rotz SJ, Ann O’riordan M, Kim C, Langer N, Cruz C, Schilz R, et al. Nocturnal hemoglobin desaturation is associated with reticulocytosis in adults with sickle cell disease and is independent of obstructive sleep apnea. Am J Hematol. 2016;91:E355–E356. doi: 10.1002/ajh.24432. [DOI] [PubMed] [Google Scholar]
- 134.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 135.Mann-Jiles V, Thompson K, Lester J. Sleep impairment and insomnia in sickle cell disease: a retrospective chart review of clinical and psychological indicators. J Am Assoc Nurse Pract. 2015;27:441–449. doi: 10.1002/2327-6924.12212. [DOI] [PubMed] [Google Scholar]
- 136.Moscou-Jackson G, Allen J, Kozachik S, Smith MT, Budhathoki C, Haywood C., Jr Acute pain and depressive symptoms: independent predictors of insomnia symptoms among adults with sickle cell disease. Pain Manag Nurs. 2016;17:38–46. doi: 10.1016/j.pmn.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Daily mood as a mediator or moderator of the pain-sleep relationship in children with sickle cell disease. J Pediatr Psychol. 2008;33:317–322. doi: 10.1093/jpepsy/jsm058. [DOI] [PubMed] [Google Scholar]
- 138.Marshall MJ, Bucks RS, Hogan AM, Hambleton IR, Height SE, Dick MC, et al. Auto-adjusting positive airway pressure in children with sickle cell anemia: results of a phase I randomized controlled trial. Haematologica. 2009;94:1006–1010. doi: 10.3324/haematol.2008.005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Warrier R, Chauhan A, Athale U. Tonsillectomy and adenoidectomy for obstructive sleep apnea in sickle cell anemia. Indian J Pediatr. 2010;77:669–672. doi: 10.1007/s12098-010-0093-2. [DOI] [PubMed] [Google Scholar]
- 140.Finch P, Stocks RM, Smeltzer MP, Kimble A, Schoumacher R, Hankins JS. Effects of adenotonsillectomy on polysomnographic parameters in children with sickle cell disease. Pediatr Blood Cancer. 2013;60:E26–E28. doi: 10.1002/pbc.24479. [DOI] [PubMed] [Google Scholar]
- 141.Farrell AN, Goudy SL, Yee ME, Leu RM, Landry AM. Adenotonsillectomy in children with sickle cell disease and obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2018;111:158–161. doi: 10.1016/j.ijporl.2018.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duke RL, Scott JP, Panepinto JA, Flanary VA. Perioperative management of sickle cell disease children undergoing adenotonsillectomy. Otolaryngol Head Neck Surg. 2006;134:370–373. doi: 10.1016/j.otohns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 143.Grady AJ, Hankins JS, Haberman B, Schoumacher R, Stocks RM. Hydroxyurea treatment effect on children with sickle cell disease and obstructive sleep apnea. Sleep Breath. 2017;21:697–701. doi: 10.1007/s11325-017-1458-9. [DOI] [PubMed] [Google Scholar]
- 144.Narang I, Kadmon G, Lai D, Dhanju S, Kirby-Allen M, Odame I, et al. Higher nocturnal and awake oxygen saturations in children with sickle cell disease receiving hydroxyurea therapy. Ann Am Thorac Soc. 2015;12:1044–1049. doi: 10.1513/AnnalsATS.201410-473OC. [DOI] [PubMed] [Google Scholar]
- 145.Singh SA, Koumbourlis AC, Aygun B. Resolution of chronic hypoxemia in pediatric sickle cell patients after treatment with hydroxyurea. Pediatr Blood Cancer. 2008;50:1258–1260. doi: 10.1002/pbc.21480. [DOI] [PubMed] [Google Scholar]
- 146.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365:44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 147.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39:112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 149.Niss O, Quinn CT, Lane A, Daily J, Khoury PR, Bakeer N, et al. Cardiomyopathy with restrictive physiology in sickle cell disease. JACC Cardiovasc Imaging. 2016;9:243–252. doi: 10.1016/j.jcmg.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mehari A, Alam S, Tian X, Cuttica MJ, Barnett CF, Miles G, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187:840–847. doi: 10.1164/rccm.201207-1222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nguyen KL, Tian X, Alam S, Mehari A, Leung SW, Seamon C, et al. Elevated transpulmonary gradient and cardiac magnetic resonance-derived right ventricular remodeling predict poor outcomes in sickle cell disease. Haematologica. 2016;101:e40–e43. doi: 10.3324/haematol.2015.125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126:443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gordeuk VR, Minniti CP, Nouraie M, Campbell AD, Rana SR, Luchtman-Jones L, et al. Elevated tricuspid regurgitation velocity and decline in exercise capacity over 22 months of follow up in children and adolescents with sickle cell anemia. Haematologica. 2011;96:33–40. doi: 10.3324/haematol.2010.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paul R, Minniti CP, Nouraie M, Luchtman-Jones L, Campbell A, Rana S, et al. Clinical correlates of acute pulmonary events in children and adolescents with sickle cell disease. Eur J Haematol. 2013;91:62–68. doi: 10.1111/ejh.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Turpin M, Chantalat-Auger C, Parent F, Driss F, Lionnet F, Habibi A, et al. Chronic blood exchange transfusions in the management of pre-capillary pulmonary hypertension complicating sickle cell disease. Eur Respir J. 2018;52:52. doi: 10.1183/13993003.00272-2018. [DOI] [PubMed] [Google Scholar]
- 156.Klings ES, Anton Bland D, Rosenman D, Princeton S, Odhiambo A, Li G, et al. Pulmonary arterial hypertension and left-sided heart disease in sickle cell disease: clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol. 2008;83:547–553. doi: 10.1002/ajh.21187. [DOI] [PubMed] [Google Scholar]
- 157.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, et al. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, et al. MSH Investigators. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 159.Gordeuk VR, Campbell A, Rana S, Nouraie M, Niu X, Minniti CP, et al. Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood. 2009;114:4639–4644. doi: 10.1182/blood-2009-04-218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sokunbi OJ, Ekure EN, Temiye EO, Anyanwu R, Okoromah CAN. Pulmonary hypertension among 5 to 18 year old children with sickle cell anaemia in Nigeria. PLoS One. 2017;12:e0184287. doi: 10.1371/journal.pone.0184287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barst RJ, Mubarak KK, Machado RF, Ataga KI, Benza RL, Castro O, et al. ASSET study group. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149:426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. walk-PHaSST Investigators and Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello R, D’Ascola G, et al. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–458. [PubMed] [Google Scholar]
- 164.Minniti CP, Machado RF, Coles WA, Sachdev V, Gladwin MT, Kato GJ. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147:737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weir NA, Conrey A, Lewis D, Mehari A. Riociguat use in sickle cell related chronic thromboembolic pulmonary hypertension: a case series. Pulm Circ. 2018;8:2045894018791802. doi: 10.1177/2045894018791802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weir NA, Saiyed R, Alam S, Conrey A, Desai HD, George MP, et al. Prostacyclin-analog therapy in sickle cell pulmonary hypertension. Haematologica. 2017;102:e163–e165. doi: 10.3324/haematol.2015.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Weatherald J, Savale L, Humbert M. Medical management of pulmonary hypertension with unclear and/or multifactorial mechanisms (group 5): is there a role for pulmonary arterial hypertension medications? Curr Hypertens Rep. 2017;19:86. doi: 10.1007/s11906-017-0783-5. [DOI] [PubMed] [Google Scholar]
- 168.Villers MS, Jamison MG, De Castro LM, James AH.Morbidity associated with sickle cell disease in pregnancy Am J Obstet Gynecol 2008199125e1–5 [DOI] [PubMed] [Google Scholar]
- 169.Costa VM, Viana MB, Aguiar RA. Pregnancy in patients with sickle cell disease: maternal and perinatal outcomes. J Matern Fetal Neonatal Med. 2015;28:685–689. doi: 10.3109/14767058.2014.928855. [DOI] [PubMed] [Google Scholar]
- 170.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 171.Guyatt GH, Eikelboom JW, Gould MK, Garcia DA, Crowther M, Murad MH, et al. American College of Chest Physicians. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e185S–194S. doi: 10.1378/chest.11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Naik RP, Lanzkron S. Baby on board: what you need to know about pregnancy in the hemoglobinopathies. Hematology (Am Soc Hematol Educ Program) 2012;2012:208–214. doi: 10.1182/asheducation-2012.1.208. [DOI] [PubMed] [Google Scholar]
- 173.Boechat TdeO, do Nascimento EM, Lobo CL, Ballas SK. Deep venous thrombosis in children with sickle cell disease. Pediatr Blood Cancer. 2015;62:838–841. doi: 10.1002/pbc.25431. [DOI] [PubMed] [Google Scholar]
- 174.Cavo M, Wang W, O’Brien SH. Use of low molecular weight heparin for thromboprophylaxis in a pediatric inpatient population: reasons for use and incidence of bleeding complications. Thromb Res. 2010;125:370–372. doi: 10.1016/j.thromres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 175.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 176.Brunson A, Lei A, Rosenberg AS, White RH, Keegan T, Wun T. Increased incidence of VTE in sickle cell disease patients: risk factors, recurrence and impact on mortality. Br J Haematol. 2017;178:319–326. doi: 10.1111/bjh.14655. [DOI] [PubMed] [Google Scholar]
- 177.Strouse JJ, Lobner. K, Lanzkron S, Haywood C Jr. NIH and National Foundation expenditures for sickle cell disease and cystic fibrosis are associated with PubMed publications and FDA approvals. Blood. 2013;122:1739. [Google Scholar]
- 178.Caboot JB, Allen JL. Hypoxemia in sickle cell disease: significance and management. Paediatr Respir Rev. 2014;15:17–23. doi: 10.1016/j.prrv.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 179.Caboot JB, Jawad AF, McDonough JM, Bowdre CY, Arens R, Marcus CL, et al. Non-invasive measurements of carboxyhemoglobin and methemoglobin in children with sickle cell disease. Pediatr Pulmonol. 2012;47:808–815. doi: 10.1002/ppul.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Raj A, Bertolone S, Bond S, Burnett D, Denker A. Cathlink 20: a subcutaneous implanted central venous access device used in children with sickle cell disease on long-term erythrocytapheresis--a report of low complication rates. Pediatr Blood Cancer. 2005;44:669–672. doi: 10.1002/pbc.20252. [DOI] [PubMed] [Google Scholar]
- 181.Piccin A, Murphy C, Eakins E, Kinsella A, McMahon C, Smith OP, et al. Protein C and free protein S in children with sickle cell anemia. Ann Hematol. 2012;91:1669–1671. doi: 10.1007/s00277-012-1447-9. [DOI] [PubMed] [Google Scholar]
- 182.Kucuk O, Gilman-Sachs A, Beaman K, Lis LJ, Westerman MP. Antiphospholipid antibodies in sickle cell disease. Am J Hematol. 1993;42:380–383. doi: 10.1002/ajh.2830420409. [DOI] [PubMed] [Google Scholar]
- 183.Noubouossie DF, Lê PQ, Corazza F, Debaugnies F, Rozen L, Ferster A, et al. Thrombin generation reveals high procoagulant potential in the plasma of sickle cell disease children. Am J Hematol. 2012;87:145–149. doi: 10.1002/ajh.22206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.