Abstract
Objective:
In this study, we explored the effect of zinc supplementation on markers of inflammation and monocyte activation in ART-treated HIV infection.
Methods:
This is a phase I open labeled randomized double arm study, exploring the efficacy and safety of zinc supplementation on inflammation in ≥ 18 years old people living with HIV (PLHIV) in the US, on stable ART and with zinc levels ≤75 μg/dL in the last 60 days. Patients were randomized 1:1 to zinc gluconate capsules at a dose of 45 mg (low-dose), or 90 mg (high-dose) elemental zinc daily for 16 weeks. We assessed inflammatory and gut integrity biomarkers at baseline and 16 weeks.
Results:
Overall, a total of 52 participants were enrolled (25 participants in the low-dose arm and 27 participants in the high-dose arm). Median (IQR) age was 49 (38, 60) years, 77% were males and 73% were African Americans. At baseline, median zinc levels were 73 (64, 86) μg/dL. Median circulating zinc levels increased to 91 μg/dL in the low-dose arm and to 100 μg/dL in the high-dose arm. Overall, 48–60% of participants experiencing a reduction in biomarkers levels. The margin of reduction ranged between 8 and 21%. This change was meaningful with large effect size (Cohen’s ranging from 5–19).
Conclusion:
In this pilot study we found that zinc supplementation is effective at increasing circulating zinc levels. In addition, our findings provide novel data suggesting that zinc can impact a biological signature in PLHIV and modulate biomarkers associated with clinical comorbidities.
Keywords: HIV, zinc, inflammation, immune activation, morbidity, gut integrity
Introduction:
The WHO estimates that nearly two billion individuals may be zinc deficient in resource limited settings. Prior to effective ART, zinc deficiency was prevalent in adults living with HIV (PLHIV) [1–3]. Low plasma zinc levels have been linked with disease progression, [4]. Unlike what is observed with most nutrients, the prevalence of zinc deficiency appears to be high, even after participants are treated with ART [3].
Studies of PLHIV show that although ART decreases inflammation[5], inflammatory markers remain elevated and increasing evidence suggests that chronic inflammation plays an important role in cardiovascular disease (CVD).
Zinc deficiency may be associated with diseases, similarly to HIV, that are associated with ongoing inflammation such as rheumatoid arthritis, diabetes and atherosclerosis[6–8]. Zinc homeostasis is important for several aspects of the immune system, however, there is a lack of data related to the effect of zinc status on the heightened inflammation and monocyte activation state in HIV.
The current study is an open labeled randomized double arm study, assessing the efficacy and safety of zinc supplementation for 16 weeks in PLHIV, who were on stable ART, and had documented zinc deficiency. Our primary objective was to examine the effect of zinc on inflammation markers in this population; secondary objectives were to assess the effect of the intervention more specifically on monocyte activation, gut epithelial barrier dysfunction and microbial translocation that have been associated with co-morbidities and increased mortality in HIV [9–12]
Methods
Study Design
This is a pilot open labeled randomized double arm study. Participants were prospectively enrolled at University Hospitals Case Medical Center, Cleveland, Ohio. The study was approved by the local Institutional Review Board, and written informed consent was provided by all participants (ClinicalTrials.gov Identifier: ). All participants were ≥18 years of age, with HIV-1 infection on stable ART for at least 3 months with cumulative ART duration of at least 6 months, HIV-1 RNA <400 copies/mL in the last 4 months prior to study entry. Participants were excluded if they were pregnant, lactating, or had an active infectious or inflammatory condition. Uncontrolled diabetes and known cardiovascular diseases were excluded. We focused on participants with documented zinc deficiency, as defined by participants having serum zinc levels ≤75 μg/dL in the last 60 days.
Zinc supplementation
The Recommended Dietary Allowance for zinc is an intake of 11 mg zinc/d for men and 8 mg zinc/d women[13]. Normal serum zinc levels range between 75–150 μg/dL. We have used 45 mg and 90 mg elemental zinc/d as oral supplementation because it has been used safely in non-HIV studies for 1 year duration, and at this concentration, with no serious adverse events [14]. We used the zinc gluconate as the form most commonly used and with the most available safety data in clinical trials outside of HIV[14–20].
Randomization and blinding
The randomization schedule was performed by a statistician using SAS software to create a list based on permuted variably sized block randomization. Participants were randomized by the study pharmacist to gluconate capsules at a dose of 45 mg (low-dose), or 90 mg (high-dose), elemental zinc daily.
Patients either took 1 (45mg) versus 2 (90mg) zinc gluconate capsules. The research assistant, the principle investigator, and all laboratory personnel performing the study assays were blinded to treatment assignment.
Primary and secondary outcomes
At entry, and week 16, fasting (for at least 8 hours) blood draws were obtained and plasma and serum stored for measurement of a marker of monocyte activation soluble CD14 (sCD14), of systemic inflammation [high sensitivity C reactive protein (hsCRP) and soluble tumor necrosis alpha receptor I and II (sTNFR-I and II)] which were measured by ELISA (R &D Systems, Minneapolis, Minnesota, USA and ALPCO, Salem, New Hampshire, USA and Mercodia, Uppsala, Sweden)., Lipopolysaccharide binding protein (LBP), a marker of microbial translocation (Hycult Biotech Inc. PA) and a marker of gut integrity, intestinal fatty acid binding protein (IFABP, R &D Systems, Minneapolis, Minnesota, USA)were measured by ELISA [21–23]. The intra and inter-assay variability were less than 10% for all markers. All assays were done at Dr Funderburg’s laboratory at Ohio State University, Columbus, OH.
At entry, weeks 4, 10 and 16, zinc adherence and adverse events were assessed.
The DSMB met every 6 months to review safety data and adverse events.
Statistical analysis
The primary objective of this pilot study was to assess the safety and tolerability of zinc in PLHIV. The secondary outcomes were to demonstrate the impact on the biological signatures in PLHIV (markers of inflammation, monocyte activation, coagulation and microbial translocation).
First, we presented summary statistics for most of the relevant biomarkers and demographic characteristics measured at the baseline. We compared various markers and characteristics for the two groups of participants using the Wilcoxon rank sum tests, Chi-square tests, and Fisher’s exact tests, as appropriate. Next, we presented the changes of the zinc levels over the study period using Box plots for the two arms of the study. We calculated the percentages of participants who experienced a biomarker reduction to evaluate the impact of zinc supplementation. To assess the meaningful change in biological signatures, we performed significance tests for the shift of distributions of the selected biomarkers after the zinc supplementation using Kolmogorov-Smironow (K-S) test. The amount of shift or effect size was measured using Cohen’s D value. All statistical analyses for the this study were conducted using statistical software Stata 15.0 [24] and R 3.4.0.
Results
Baseline characteristics
A total of 52 participants were enrolled (25 participants in the low-dose arm and 27 participants in the high-dose arm). Baseline characteristics are shown in Table 1. Median (IQR) age was 49 (38, 60) years, 77% were males and 73% were African Americans. Participants in the low-dose arm were significantly older and had longer duration of ART. At baseline, median zinc levels were 73 (64, 86) μg/dL.
Table 1:
Baseline characteristics
| Low-dose (45 mg) arm Median[IQR]/Frequency(%)a |
High-dose (90mg) arm Median[IQR]/Frequency(%) |
p-valueb | |
|---|---|---|---|
| No. of participants (n) | 25 | 27 | |
| Age | 54.00 [48.00, 60.00] | 47.00 [38.00, 54.50] | 0.01* |
| Male (%) | 20 (80.00) | 20 (74.07) | 0.75 |
| African American (%) | 18 (72.00) | 20 (74.07) | 1.00 |
| Current smoking (%) | 11 (44.00) | 16 (59.26) | 0.41 |
| Alcohol (%) | 15 (60.00) | 20 (76.92) | 0.24 |
| Family history of myocardial infarctions (%) | 9 (36.00) | 8 (29.63) | 0.77 |
| BMI (kg/m2) | 24.93 [21.84, 30.24] | 26.58 [23.57, 29.59] | 0.36 |
| Waist hip ratio | 0.91 [0.88, 0.95] | 0.91 [0.88, 0.96] | 0.79 |
| Systolic blood pressure (mm Hg) | 132.00 [119.00, 145.00] | 131.00 [120.00, 140.00] | 0.52 |
| Diastolic blood pressure (mm Hg) | 82.00 [77.00, 91.00] | 83.00 [77.00, 89.00] | 0.99 |
| Albumin (g/dL) | 4.10[3.90, 4.20] | 4.30[4.10, 4.60] | 0.02* |
| HDL cholesterol (mg/dL) | 43.80 [36.50, 58.40] | 49.10 [43.80, 62.33] | 0.26 |
| LDL cholesterol (mg/dL) | 88.00 [73.00, 107.00] | 92.50 [82.25, 114.00] | 0.27 |
| CD4 cell count (cells/μL) | 685.00 [547.00, 983.00] | 681.00 [456.50, 1007.50] | 0.71 |
| HIV RNA(<20 copies) (%) | 19 (76.00) | 24 (88.89) | 0.28 |
| ART duration (months) | 138.83 [121.32, 185.93] | 104.40 [47.70, 145.45] | 0.01* |
| Protease inhibitor use (%) | 7 (28.00) | 4 (15.38) | 0.32 |
| hsCRP (ng/mL) | 2433.21 [1048.94, 4360.62] | 2323.83 [1292.22, 4929.42] | 0.43 |
| sCD14 (pg/mL) | 1829.53 [1646.91, 2193.73] | 1567.95 [1338.99, 1936.94] | 0.08 |
| sTNF-RI (pg/mL) | 1047.80 [938.75, 1368.11] | 1026.55 [797.81, 1228.81] | 0.28 |
| sTNF-RII (pg/mL) | 3349.42 [2717.60, 4130.24] | 3052.59 [2458.79, 3373.41] | 0.13 |
| LBP (μg/mL) | 15025.92 [10921, 18306] | 15482.88 [10948, 20870] | 0.50 |
| IFABP (pg/mL) | 3445.31 [2621.04, 4701.49] | 3313.70 [1723.43, 4447.83] | 0.43 |
| Zinc (μg/dL) | 74.00 [64.00, 83.00] | 73.00 [66.00, 86.00] | 0.96 |
All continuous variables are summarized as median [1st quantile, 3rd quantile]. For normally distributed variables, median is equal to mean.
The Wilcoxon rank sum tests are used for continuous variables. Chi-square tests and Fisher’s exact tests are used for categorical variables as appropriate.
p<0.05.
Zinc safety and changes over time
After 16 weeks, loss to follow up was minimal with 94% retention. There was 1 loss to follow up in the low-dose arm and 3 participants lost to follow up in the high-dose arm. There was one possible study related grade 3 adverse event: a patient who developed nausea and abdominal cramps 6 days after initiating high-dose zinc supplement.
Median circulating zinc levels increased from 74 to 91 μg/dL in the low-dose arm and from 73 to 100 μg/dL in the high-dose arm.
In addition, 88% of participants in the low-dose arm and 96% in the high-dose arm reached zinc levels >75 μg/dL.
Impact on biological signature
Overall, 48–60% of participants experienced a reduction in biomarkers in the combined arms (Figure 1). There was a larger proportion of participants with reduction in sCD14 and IFABP in the participants in the low-dose arm, however more participants had decreases in LBP and sTNF-RI in the high-dose arm. Biomarker reductions were meaningful with Cohen’s D ranging from 5.25–19.44.
Figure 1:
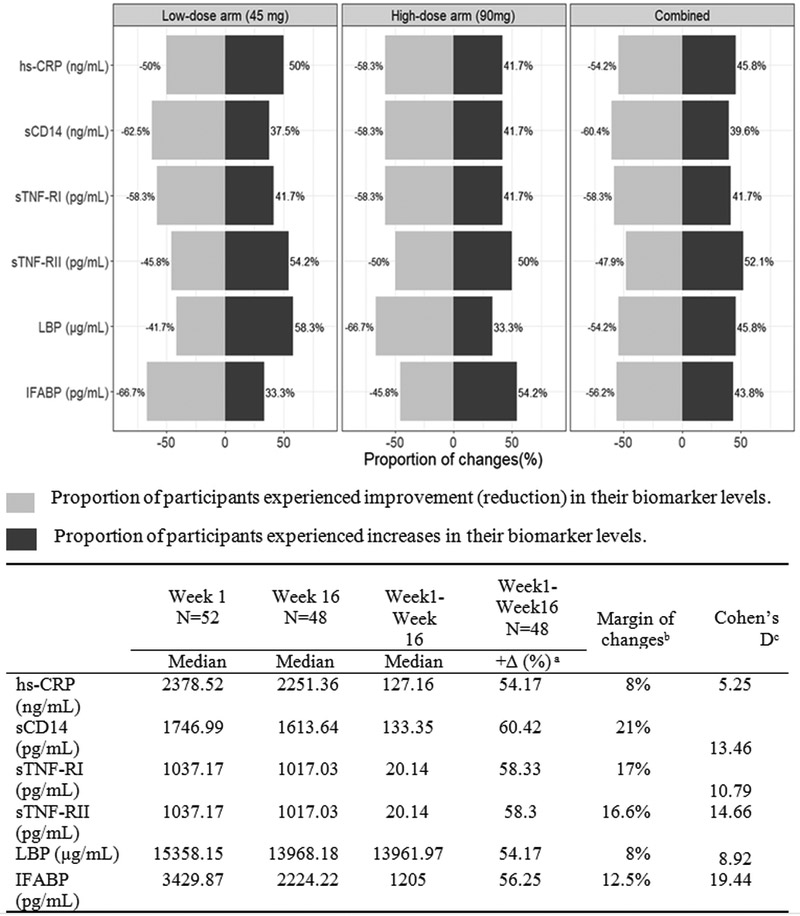
The proportion of participants with biomarker changes
a.+∆ (%) indicates the percentages of participants experienced decrease in the biomarker. Margin of changes calculated as percent of positive change minus percent of negative change.
b.The Cohen’s d is calculated after Box-Cox transformation. Small effect ≤ 0.2; Medium Effect [0.3, 0.8]; Large Effect > 0.8.
Discussion
In this pilot study, we found that in PLHIV on effective ART, zinc supplementation is safe, effective at increasing zinc levels and decreasing biomarkers known to be elevated in this population and associated with clinical comorbidities.
Zinc deficiency
Zinc is an essential nutrient and is involved in vital pathways including protein synthesis and gene transcription[25]. Zinc deficiency is the 5th leading cause for loss of healthy life years[26]. In the Nutrition for Healthy Living (NFHL) study, 40% of men and 36% of women on ART had low zinc levels, and participants in the upper quartiles of zinc had lower HIV viral load levels than those in the lowest quartile[3]. Mean zinc levels in PLHIV from different cohorts range between 60–75 μg/dL[3, 27, 28]. In previous zinc supplementation trials performed in PLHIV, the participants were either not on ART, or not virally suppressed [27–32]. In this study, we assessed for the first time in HIV, the effects of zinc supplementation in participants virally suppressed on effective ART with low baseline zinc levels. We found that zinc supplementation at both high and low-doses is well tolerated with no serious toxicity. Importantly we also found that 45 mg and 90 mg of elemental zinc daily are enough in PLHIV to raise serum zinc levels above 75 μg/dL in most.
Zinc and inflammation
Zinc deficiency may be associated with diseases, similarly to HIV, that are associated with ongoing inflammation such as rheumatoid arthritis, diabetes and atherosclerosis[6–8]. Zinc has known anti-oxidative and anti-inflammatory properties[33] and is involved in immune regulation including cell maturation, cell differentiation, and apoptosis[34]. Zinc homeostasis is important for several aspects of the immune system. Zinc deficiency results in an increased sensitivity to the effects of oxidative stress. As previously noted, no studies have investigated the relationship between zinc status and systemic inflammation in ART-treated PLHIV. We measured soluble CD14, a marker of monocyte activation in response to microbial translocation, because it has been linked to mortality in HIV[35] and by our group, to coronary calcification and subclinical vascular disease[36]. The other markers of systemic inflammation were chosen because they have predicted HIV comorbidities[37–39], and are relevant to CVD research.
In our study, we found that zinc supplementation in PLHIV with zinc deficiency can decrease markers of systemic inflammation, monocyte activation and microbial translocation in some cases in more than half of the participants. Although the margin of reduction in the biomarkers after zinc supplementation did not surpass 21%, the quantitative measure of the magnitude of change represented by Cohen’s d (the difference of the means divided by the standard deviation) is large for all biomarkers. We hypothesize that the size of the reduction is likely secondary to the short period of supplementation in our study.
Zinc and intestinal barrier
In PLHIV, the causes of the heightened immune activation remain unclear, but one of the key contributors is likely gut epithelial barrier dysfunction and microbial translocation[40]. The benefits of zinc supplementation during diarrheal episodes, in areas with high prevalence of zinc deficiency, are well documented [41]. Dietary zinc deficiency causes impairment of gut architecture, such as shorter and narrower jejunal villi, reduced absorptive surface area, decreased number of mitochondria, swelling of the endoplasmic reticulum and atrophic Golgi apparatus, accompanied by increased membrane permeability and declined cell mobility[42]. In this study, we measured IFABP a marker of enterocyte damage as IFABP leaks out of damaged small intestine epithelial cells[43]. We also measured LPB, which is produced by hepatocytes in response to the presence lipopolysaccharide (LPS) and other bacterial products in the portal venous system. The differences seen in the levels of LBP and IFABP between the two arms was unexpected. We suspect, however, that the regulation of the intestinal barrier is likely a complex process and immune and dietary factors both modulate the barrier function. Our findings support our hypothesis that zinc supplementation in ART-treated HIV infection has the potential to be an effective agent in decreasing microbial translocation and improving gut epithelial barrier dysfunction. Further studies are needed to determine zinc’s role on the intestinal barrier in HIV, and whether its effect is dose dependent.
Our study has several limitations. Due to the small sample size and short study period, we cannot establish causation. We did not include a placebo control group for comparison. Our study focused on participants with treated HIV and documented zinc deficiency in the United States, therefore our findings cannot be generalized to different populations or to resource limited settings. We did not obtain dietary or physical activity patterns. Lastly, the participants in the high-dose arm were significantly younger than the participants in the low-dose arm and with shorter ART duration which may have affected our findings.
This is the first study to investigate zinc supplementation in PLHIV with viral suppression and zinc deficiency. Studying the relationship between zinc and inflammation in PLHIV is an innovative approach to prevention of co-morbidities as opposed to only treatment of established disease. In addition, we offer potential mechanistic pathways for the relationship between zinc and systemic inflammation by demonstrating a potential role of zinc on gut epithelial barrier dysfunction and result microbial translocation
Larger studies are crucial to investigate the role of zinc supplementation in HIV comorbidities linked to inflammation.
Acknowledgements
The authors would like to thank the patients who participated in this research.
Source of support:
This work was supported by a grant from NCCIH (AT009153) to GM. Additional support was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to SDF (K23HD088295–01A1).
Conflicts of Interest and Source of Funding: GAM served as a consultant for Gilead, BMS, GSK/Viiv, and Merck, and has received research funding from Gilead, Merck, GSK/Viiv, and BMS. NF serves as a consultant for Gilead. All other authors had no conflict of interest.
This work was supported by a grant from NCCIH (AT009153) to GM. Additional support was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to SDF (K23HD088295–01A1).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Preliminary findings were presented at the Conference for Retroviruses and Opportunistic Infections, Seattle, Washington, March 2019.
REFERENCES
- 1.Baum MK, Shor-Posner G, Lu Y, Rosner B, Sauberlich HE, Fletcher MA, et al. Micronutrients and HIV-1 disease progression. Aids 1995; 9(9):1051–1056. [DOI] [PubMed] [Google Scholar]
- 2.Baum MK, Javier JJ, Mantero-Atienza E, Beach RS, Fletcher MA, Sauberlich HE, et al. Zidovudine-associated adverse reactions in a longitudinal study of asymptomatic HIV-1-infected homosexual males. Journal of acquired immune deficiency syndromes 1991; 4(12):1218–1226. [PubMed] [Google Scholar]
- 3.Jones CY, Tang AM, Forrester JE, Huang J, Hendricks KM, Knox TA, et al. Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the Nutrition for Healthy Living cohort. Journal of acquired immune deficiency syndromes 2006; 43(4):475–482. [DOI] [PubMed] [Google Scholar]
- 4.Falutz J, Tsoukas C, Gold P. Zinc as a cofactor in human immunodeficiency virus-induced immunosuppression. Jama 1988; 259(19):2850–2851. [DOI] [PubMed] [Google Scholar]
- 5.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Current opinion in HIV and AIDS 2014; 9(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmunity reviews 2015; 14(4):277–285. [DOI] [PubMed] [Google Scholar]
- 7.Chabosseau P, Rutter GA. Zinc and diabetes. Archives of Biochemistry and Biophysics 2016; 611:79–85. [DOI] [PubMed] [Google Scholar]
- 8.Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology 2006; 7(5–6):421–428. [DOI] [PubMed] [Google Scholar]
- 9.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. The Journal of infectious diseases 2014; 210(8):1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases 2011; 203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. The Journal of infectious diseases 2012; 206(10):1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks MA, Rabkin CS, Engels EA, Busch E, Kopp W, Rager H, et al. Markers of microbial translocation and risk of AIDS-related lymphoma. Aids 2013; 27(3):469–474. [DOI] [PubMed] [Google Scholar]
- 13.Institue of MEdicine FaNB. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press; 2001. [PubMed] [Google Scholar]
- 14.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. The American journal of clinical nutrition 2010; 91(6):1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr 2001; 20(3):212–218. [DOI] [PubMed] [Google Scholar]
- 16.Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr 2003; 22(4):316–321. [DOI] [PubMed] [Google Scholar]
- 17.Silk R, LeFante C. Safety of zinc gluconate glycine (Cold-Eeze) in a geriatric population: a randomized, placebo-controlled, double-blind trial. Am J Ther 2005; 12(6):612–617. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Ahn J. Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res 2014; 157(2):101–106. [DOI] [PubMed] [Google Scholar]
- 19.Shah UH, Abu-Shaheen AK, Malik MA, Alam S, Riaz M, Al-Tannir MA. The efficacy of zinc supplementation in young children with acute lower respiratory infections: a randomized double-blind controlled trial. Clin Nutr 2013; 32(2):193–199. [DOI] [PubMed] [Google Scholar]
- 20.Seet RC, Lee CY, Lim EC, Quek AM, Huang H, Huang SH, et al. Oral zinc supplementation does not improve oxidative stress or vascular function in patients with type 2 diabetes with normal zinc levels. Atherosclerosis 2011; 219(1):231–239. [DOI] [PubMed] [Google Scholar]
- 21.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. The Journal of infectious diseases 2014; 209(8):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014; 58(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburg NT, Boucher M, Sattar A, Kulkarni M, Labbato D, Kinley BI, et al. Rosuvastatin Decreases Intestinal Fatty Acid Binding Protein (I-FABP), but Does Not Alter Zonulin or Lipopolysaccharide Binding Protein (LBP) Levels, in HIV-Infected Subjects on Antiretroviral Therapy. Pathogens & immunity 2016; 1(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 14. In. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 25.Maxfield L, Crane JS. Zinc Deficiency In: StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC; 2018. [Google Scholar]
- 26.Wessels I, Maywald M, Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017; 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green JA, Lewin SR, Wightman F, Lee M, Ravindran TS, Paton NI. A randomised controlled trial of oral zinc on the immune response to tuberculosis in HIV-infected patients. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2005; 9(12):1378–1384. [PubMed] [Google Scholar]
- 28.Carcamo C, Hooton T, Weiss NS, Gilman R, Wener MH, Chavez V, et al. Randomized controlled trial of zinc supplementation for persistent diarrhea in adults with HIV-1 infection. Journal of acquired immune deficiency syndromes 2006; 43(2):197–201. [DOI] [PubMed] [Google Scholar]
- 29.Asdamongkol N, Phanachet P, Sungkanuparph S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV-infected patients with immunological discordance after antiretroviral therapy. Japanese journal of infectious diseases 2013; 66(6):469–474. [DOI] [PubMed] [Google Scholar]
- 30.Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010; 50(12):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson L, Thacher TD, Yassin MA, Onuoha NA, Usman A, Emenyonu NE, et al. Randomized controlled trial of zinc and vitamin A as co-adjuvants for the treatment of pulmonary tuberculosis. Tropical medicine & international health : TM & IH 2010; 15(12):1481–1490. [DOI] [PubMed] [Google Scholar]
- 32.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. The British journal of nutrition 2006; 95(4):762–770. [DOI] [PubMed] [Google Scholar]
- 33.Lee SR. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative medicine and cellular longevity 2018; 2018:9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haase H, Rink L. Multiple impacts of zinc on immune function. Metallomics : integrated biometal science 2014; 6(7):1175–1180. [DOI] [PubMed] [Google Scholar]
- 35.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28(7):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009; 49(7):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McComsey GA, Kitch D, Sax PE, Tierney C, Jahed NC, Melbourne K, et al. Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr 2014; 65(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hileman CO, Labbato DE, Storer NJ, Tangpricha V, McComsey GA. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS 2014; 28(12):1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assimakopoulos SF, Dimitropoulou D, Marangos M, Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection 2014. [DOI] [PubMed] [Google Scholar]
- 41.Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. The Cochrane database of systematic reviews 2016; 12:Cd005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Southon S, Livesey G, Gee JM, Johnson IT. Intestinal cellular proliferation and protein synthesis in zinc-deficient rats. Br J Nutr 1985; 53(3):595–603. [DOI] [PubMed] [Google Scholar]
- 43.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36(7):529–535. [DOI] [PubMed] [Google Scholar]


