A 48-year-old woman with a history of recurrent pericarditis presented with 3 weeks of progressive chest pain and dyspnea. Her chest pain was pleuritic, non-positional, and similar in character to her prior episodes of pericarditis, but was atypical in that it was constant, severe in intensity, and associated with palpitations and dyspnea at rest. She denied orthopnea, paroxysmal nocturnal dyspnea, fevers, chills, or cough. She reported several days of intermittent lightheadedness without syncope.
Dr. Olenchock: Acute and chronic pericarditis often differ in etiology. Acute pericarditis can be precipitated by viruses, malignancy, mycobacteria and systemic rheumatic disease. Many cases are idiopathic and ¼ will go on to have recurrent, idiopathic pericarditis. More cases of recurrent or chronic pericarditis are secondary to rheumatic conditions. It is important to distinguish idiopathic recurrent pericarditis from serositis due to systemic rheumatic conditions, particularly Systemic Lupus Erythematosus (SLE) or drug-induced Lupus. In this patient, I would also consider sequelae of acute and recurrent pericarditis, such as constrictive pericarditis or pericardial effusion with tamponade, along with manifestations of a possible connective tissue disorder including pleural effusion, pulmonary embolism, vasculitis and valvular disease such as Libman-Sacks endocarditis in association with SLE.
She complained of longstanding bilateral shoulder and knee pain without joint erythema or swelling. She denied dry mouth or eyes but endorsed periodic mouth ‘sores’ that accompanied her prior episodes of pericarditis; these had previously been treated with antibiotics, and she was under the care of a dentist for gingival disease. She had a purpuric lower extremity rash one year prior, now resolved. She denied dysphagia, heartburn, dysuria, urgency, polyuria, weakness, or proximal muscle pain. She denied weight loss but noted occasional nausea and poor appetite. Home medications included ibuprofen, prednisone 10 mg daily, and alprazolam as needed for anxiety. She smoked 2–5 cigarettes daily and occasional marijuana but denied illicit and alcohol intake.
Prior evaluation of her recurrent pericarditis had demonstrated a positive antinuclear antibody (1:640), erythrocyte sedimentation rate 96 mm/hr (normal <20mm/hr) and C-reactive protein of 170 mg/L (normal <3 mg/L). Other serologic markers, including rheumatoid factor, anti-CCP antibodies, double stranded DNA, complement 3 and complement 4, anti-neutrophil cytoplasmic antibodies, anti-smith, anti-RNP, cryoglobulins, anti-Ro, anti-La and anti-scl-70 were negative. Previous biopsy of a lower extremity rash was notable only for extravasated red blood cells, and interpreted as non-specific, though possibly consistent with resolving vasculitis. An echocardiogram performed during an admission for pericarditis had been completely normal. She had received ibuprofen, colchicine, and multiple courses of steroids for recurrent pericarditis.
Dr. Olenchock: Oral lesions and joint pain, in the setting of associated rash and pericarditis, as well as her positive ANA, could lend itself to a diagnosis of SLE. However, it is important to evaluate for other causes of these symptoms because it is very possible to meet criteria without having SLE. Other possible rheumatic explanations for her symptomatology include seronegative rheumatoid arthritis or systemic sclerosis. HIV should be ruled out, considering her poor appetite and oral lesions.
On examination, she was afebrile, with heart rate 120 beats per minute, blood pressure 93/48 mmHg, respirations 18 breaths per minute, and oxygen saturation 97% without supplemental oxygen. Body mass index (BMI) was 23 kg/m2. She was lethargic and thin, with temporal wasting. She spoke in 2–3-word sentences and was using accessory respiratory muscles. Oral examination noted poor dentition and small blood-filled vesicles in her posterior oropharynx. Her neck was supple with no cervical lymphadenopathy. Cardiac examination revealed a regular rhythm, a loud pulmonic component of S2, right-sided S3, and a holosystolic murmur that increased with inspiration. Her jugular venous pressure was 15 cm of H2O and increased with inspiration. Carotid pulses were 1+. Pulmonary examination was without adventitious lung sounds. There was trace bilateral pitting edema. Her joint examination was unremarkable. Skin examination was notable for discrete, non-blanching, purpuric, circular macules on her bilateral dorsal arms in a perifollicular distribution. There was no rash of the lower extremities.
Dr. Olenchock: Tachycardia, borderline systemic blood pressures, elevated venous pressures, and low-volume central pulses all raise concern for cardiogenic shock. Increased P2, right-sided S3, RV heave and enhancement of systolic murmur with inspiration, also known as Carvallo’s sign, also suggest right heart failure and raise possibility of pulmonary hypertension. I would obtain an echocardiogram. Laboratory studies should be obtained to determine if there is evidence of multiorgan system failure. Blood blisters in the mouth – angina bullosa hemorrhagica – generally occur on the buccal mucosa secondary to trauma, and are not associated with systemic symptoms, although systemic rheumatic disease and infections (e.g., human immunodeficiency virus, coxsackie virus, and herpes simplex virus) have a range of oral manifestations. Several vitamin deficiencies can cause bleeding diathesis including vitamin K or C deficiency and even B12 or folate, if severe enough to cause thrombocytopenia. Vitamin C deficiency could also explain poor dentition and purpuric rashes, though these are non-specific. In the context of bleeding oral lesions, cutaneous ecchymosis, and her poor appetite and cachexia, vitamin levels should be checked.
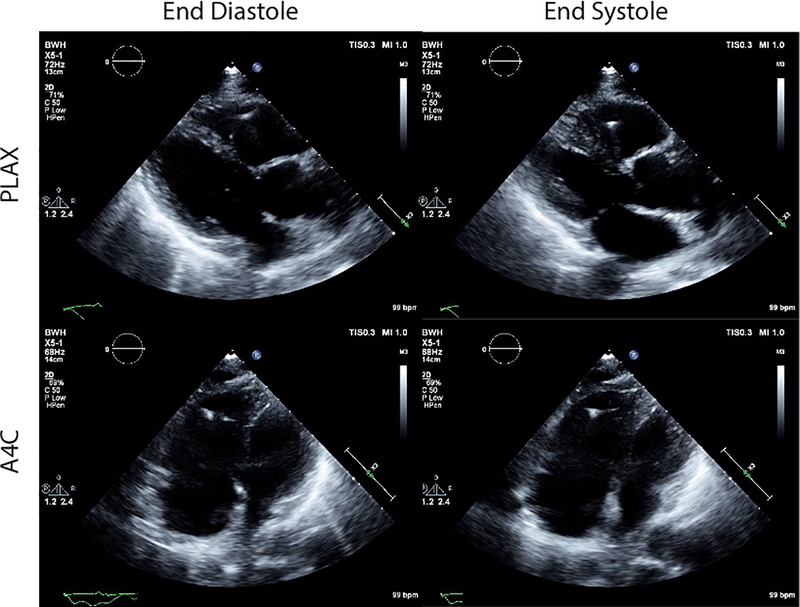
Electrocardiogram showed sinus tachycardia, right axis deviation, low voltage, and T wave inversion in leads V2-V5. A transthoracic echocardiogram demonstrated normal left ventricular function and severe right ventricular (RV) dysfunction, with systolic and diastolic septal flattening (Figure 1); estimated RV systolic pressure was 84 mmHg, with severe tricuspid regurgitation and moderate pulmonic insufficiency.
Figure 1:
Transthoracic echocardiogram demonstrating severe right ventricular (RV) dysfunction. Shown are still frame images at end-diastole and end-systole from parasternal long axis (PLAX) and apical 4-chamber (A4C).
Laboratory studies were notable for N-terminal pro-BNP 30,178 pg/mL (normal < 450), troponin T 0.05 ng/mL (normal < 0.01), lactate 5 mmol/L (normal <2.2 mmol/L), creatinine 1.4 mg/dL (normal 0.5–1.2 mg/dL), blood urea nitrogen 31 mg/dL (normal 6–23 mg/dL), white blood cell count 14 K/uL (normal 4.0–10.9 k/uL), hemoglobin 11.7 g/dL (normal, 11.5–16.4 g/dL), AST 138 U/L (normal 10–50 U/L), ALT 78 U/L (normal 10–50 U/L), alkaline phosphatase 171 U/L (normal 4–130 U/L), and total bilirubin 1.7 mg/dL (normal 0.0–1.0 mg/dL). Repeat antinuclear antibody was negative (<1:40). HIV testing was negative.
Dr. Olenchock: The echocardiogram is consistent with RV failure secondary to pulmonary hypertension (PH). Progression from normal to severely elevated RV systolic pressure in a 6-month period is uncommon and suggests a rapidly progressive pulmonary arterial hypertension (PAH). A right heart catheterization (RHC) will be required to confirm the diagnosis of PAH, and vasoreactivity testing may be considered if hemodynamically stable. Computed tomographic imaging of the chest should be pursued to assess for parenchymal lung disease along with angiographic assessment for thromboembolic disease. The echocardiogram makes left-sided failure highly unlikely.
The diffusely abnormal laboratory values are consistent with multisystem organ dysfunction, potentially due to poor perfusion. Measures to improve end-organ perfusion should be initiated.
The patient was started on dopamine, 5 μg/kg/min, and was taken to the catheterization laboratory for RHC. The procedure revealed the following hemodynamics: right atrium (RA) 16 mmHg, RV 54/19 mmHg, pulmonary artery (PA) 57/31 mmHg (mPAP 41), and pulmonary capillary wedge pressure (PCWP) 5 mmHg. PA O2 saturation was 49%, cardiac index (CI) was 1.9 L/min/m2, and pulmonary vascular resistance (PVR) was 1050 dyn•sec/cm5 (13 Woods units). Vasoreactivity testing was performed with inhaled nitric oxide (40 ppm) and the mPAP decreased to 34 mmHg, CI increased to 3.6 L/min/m2, and PVR decreased significantly to 410 dyn•sec/cm5 (5 Woods units). CT angiogram of the chest demonstrated enlarged central PA and no evidence of pulmonary embolism or parenchymal lung disease.
Dr. Olenchock: The RHC confirms severe PH and RV failure, with normal left-sided pressures. While severely elevated, the PA systolic pressure is lower than observed by echocardiogram. The discrepancy could reflect improved oxygenation and less hypoxic vasoconstriction, or interval worsening of RV function. The transpulmonary gradient of 36 mmHg and the PVR of 13 Woods units (normal 0.5–2.0, severe >5) are consistent with pre-capillary PAH. CI measurements confirm the clinical diagnosis of cardiogenic shock (CI <2.2 L/min/m2). The dramatically lower PVR and improved CI with vasoreactivity testing suggests a significant vasoconstrictive component.
She was transferred to the cardiac intensive care unit. Despite intravenous epoprostenol and furosemide, she continued to deteriorate. PVR remained severely elevated (789 dyn•sec/cm5, 9.9 Woods units). Norepinephrine, dobutamine, and vasopressin were required to maintain systemic mean arterial pressure. She was transitioned to inhaled epoprostenol, and over the next 72 hours had improvement in PVR to 436 dyn•sec/cm5 (5.5 Woods units) but was still requiring multiple vasoactive medications to maintain systemic blood pressure.
Dr. Olenchock: While continued attention and effort to optimize her hemodynamics is essential, the evaluation for an underlying cause of her PAH is equally critical. It is important not to lose sight of the diagnostic evaluation in critically ill patients.
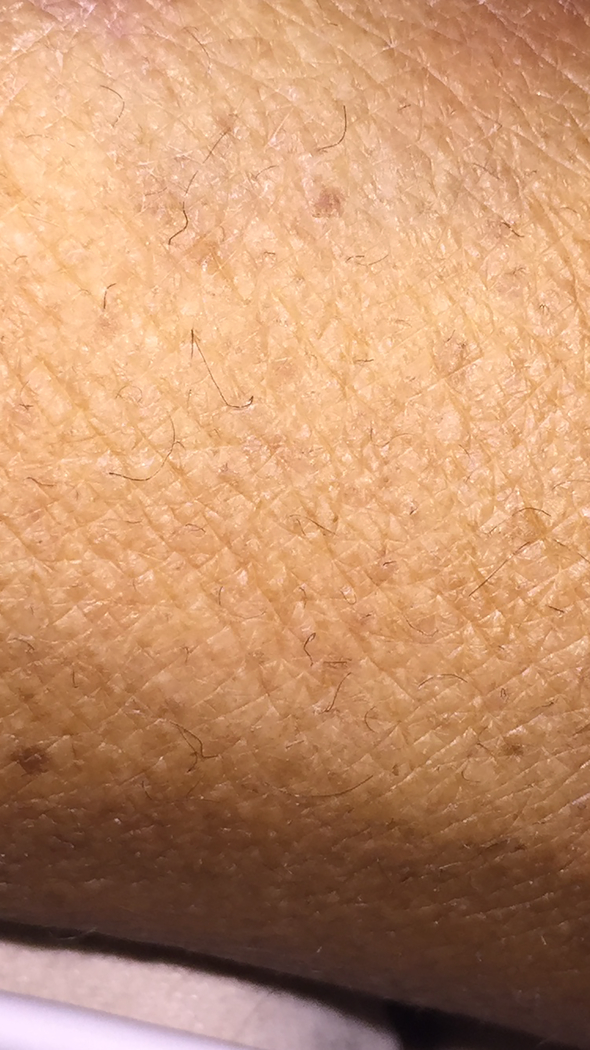
On hospital day 6, vitamin C level returned as undetectable (<0.01 mg/dL, normal 0.6–2.2), iron 18 μg/dL (normal 37–145), and a transferrin saturation 6% (normal 25–45%). On further questioning, the patient disclosed that her diet comprised of mostly coffee, chocolate bars, and iceberg lettuce. Careful physical examination identified the presence of corkscrew hairs (Figure 2).
Figure 2:
Corkscrew hairs on the patient’s forearm.
Within 24 hours of oral ascorbate, multivitamin, and intravenous iron repletion, she was weaned off vasopressors. At 36 hours, inhaled epoprostenol was discontinued and oral sildenafil initiated. By 48 hours, her symptoms had resolved. Repeat RHC demonstrated a RA pressure 17 mmHg, mPAP 26 mm Hg, PCWP 14 mm Hg, CI 5.1 L/min/m2, and PVR of 114 dyn•sec/cm5. She was discharged home on ascorbate, multivitamin and sildenafil. Repeat testing two weeks later confirmed normalization of serum ascorbate levels (94 μmol/L, normal 23–114). Repeat echocardiogram six weeks later demonstrated complete normalization of RV size and function, with estimated RV systolic pressure of 28 mmHg with only trace pulmonic and tricuspid regurgitation. Sildenafil was discontinued, and she has remained asymptomatic.
Discussion
Ascorbate (vitamin C) deficiency, also known as scurvy, is the unifying diagnosis in this case. Cardiac manifestations of scurvy were first described by Hess in infants in 19171 and it is long-known that patients eventually die from an ill-defined cardiopulmonary syndrome. More recently, rare cases of reversible pulmonary hypertension associated with ascorbate deficiency have been described2.
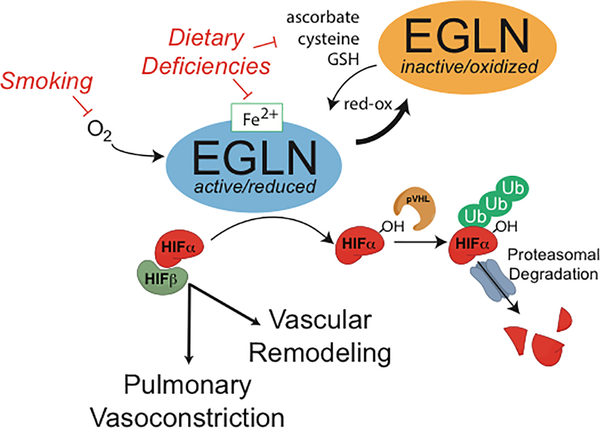
Ascorbate is a powerful reducing agent and a cofactor for many reactions catalyzed by oxygenases. Manifestations of scurvy, including bleeding, pseudovasculitic rash, arthralgia, and pericarditis are a consequence of capillary fragility due to inactivation of ascorbate-dependent oxygenases required for collagen synthesis. The EGLN family of enzymes are ascorbate-dependent oxygenases comprised of a catalytic iron (Fe II) center and that function as oxygen sensors in metazoans3,4. EGLNs hydroxylate the alpha subunit of the hypoxia inducible factor (HIF) and thereby target it for degradation. HIF is known to play an important role in hypoxic pulmonary vasoconstriction (Figure 3)3,4. Genetic data from high-altitude dwellers have connected the EGLN/HIF pathway with risk for PH3,4. In preclinical models, inactivation of EGLN1 in the pulmonary endothelium stabilizes HIF2α and results in an obliterative pulmonary vascular disease similar to idiopathic PAH5. In addition to ascorbate, reduced iron is also required as a cofactor for EGLNs and to target HIF for degradation4. Mild iron deficiency was similarly noted in other cases of reversible PH associated with Vitamin C deficiency2. EGLNs require ascorbate, reduced iron, and oxygen for full activity. Micronutrient deficiency (vitamin C and possibly iron deficiency), contributed to EGLN inactivation, HIF stabilization, and pulmonary vasoconstriction in this patient.
Figure 3:
The EGLN prolyl hydroxylase targets HIFα for proteasomal degradation. EGLN hydroxylates HIFα in an oxygen (O2) and αketoglutarate-dependent reaction. Hydroxylated HIFαis recognized by the von Hippel Lindau protein (pVHL) and targeted for polyubiquitination and proteasomal degradation. When O2 is limited, the EGLN reaction is impaired, HIFα accumulates, binds to its transcriptional binding partner HIFβ (also known as ARNT), and effects transcription of genes involved in hypoxia responses. In the lung vasculature, HIF transcriptional activity is associated with pulmonary vasoconstriction and smooth muscle cells proliferation. EGLN is oxidized and inactivated by low-frequency reactions in which αketoglutarate decarboxylation is uncoupled from HIFα hydroxylation. Reducing agents – ascorbate, cysteine, or reduced glutathione (GSH) – are required to reduce EGLN and maintain activity. Pulmonary hypertension in this patient is proposed to have resulted from dietary deficiency in these reducing agents and hypoxia from smoking, which together inactivated EGLN in the pulmonary vasculature. (HIF = hypoxia inducible factor, ARNT = aryl hydrocarbon receptor nuclear translocator, Ub = Ubiquitin, OH = hydroxyl group)
Manifestations of scurvy that mimic vasculitis, cardiac and rheumatologic disease often obscure the diagnosis. Considering the life-threatening nature of this condition and rapid reversibility with treatment, a thorough dietary history and a careful oral and mucocutaneous examination should be performed in all pulmonary hypertension patients, followed by micronutrient testing if clinically indicated. Diagnosis and repletion of vitamin C deficiency in this case fully resolved what may have otherwise been fatal.
Conclusion
This case highlights an underappreciated and potentially fatal sequelae of vitamin C deficiency. A thorough history and physical exam are critical in recognizing this often-forgotten disease, and can trigger rapidly effective, life-saving treatment.
Acknowledgements
EHP: Contributed to patient care, literature search, figures, drafting the manuscript. BAO: Contributed to patient care, literature search, figures, critical review of the manuscript. NAM: Contributed to patient care, literature search, figures, and drafting the manuscript.
Disclosures
EHP: No Disclosures. BAO: Employment; Regeneron. NAM: Research Grant; Significant; NIH National Research Service Award (NRSA).
References
- 1.Hess AF. Subacute and latent Infantile Scurvy; the cardiorespiratory syndrome (a new sign) JAMA. 1917;68:235–239. [Google Scholar]
- 2.Kupari M, Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest. 2012;142:225–227. [DOI] [PubMed] [Google Scholar]
- 3.Frise MC, Robbins PA. The pulmonary vasculature--lessons from Tibetans and from rare diseases of oxygen sensing. Exp Physiol. 2015;100:1233–1241. [DOI] [PubMed] [Google Scholar]
- 4.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. [DOI] [PubMed] [Google Scholar]
- 5.Dai Z, Li M, Wharton J, Zhu MM, Zhao YY. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2alpha. Circulation. 2016;133:2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]