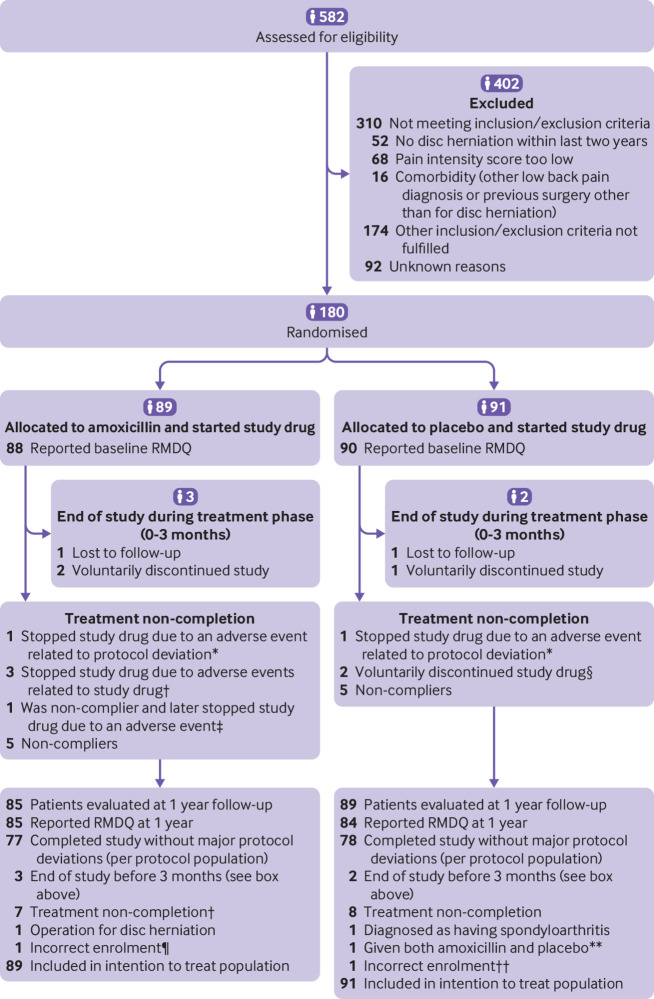
Fig 1.
Flowchart showing trial group assignments, loss to follow-up, treatment completion, and protocol deviations. RMDQ=Roland-Morris Disability Questionnaire. *One patient in amoxicillin group and one patient in placebo group became pregnant (protocol deviation because all patients were instructed to use contraception), not included in per protocol population. †Three patients in amoxicillin group stopped study drug because of adverse events and were included in per protocol population. ‡One patient in amoxicillin group stopped study drug because of adverse events but was not included in per protocol population owing to poor compliance before stopping study drug. §Two patients in placebo group discontinued because they started three month treatment with amoxicillin plus clavulanic acid. ¶Treated with apocillin seven days before randomisation. **Because of a mistake at pharmacy, patient was given a mix of bottles containing amoxicillin and placebo. ††Treated with cephalexin seven days before randomisation.