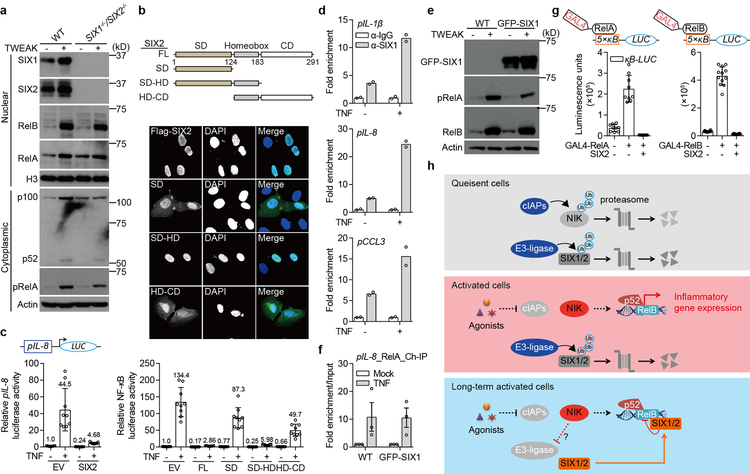
Extended Data Fig. 6. SIX-family proteins inhibit NF-κB activation through occupying the target gene promoters.
a, In order to narrow down the possible mechanisms for SIX-proteins inhibition of inflammatory gene expression, we monitored the activation, processing and nuclear translocation of RelA and RelB upon cellular stimulation with TWEAK. WT and SIX1−/− SIX2−/− H1792 cells were treated with mock or 50 ng/ml TWEAK for 24 hours. Cells lysates from cytoplasmic or nuclear fractions were analyzed by western blot. Neither SIX1 nor SIX2 blocked RelA phosphorylation, p100/52 processing, or restricted NF-κB translocation to the nucleus. b, Domain analysis of SIX-family protein function. SIX-proteins are composed of a Six Domain (SD), Homeobox domain (HD), and Coiled Coil (CD) region (diagram). Full-length SIX1 and SIX2 have 80% identical amino acids over the entirety of the protein coding sequence. The SD and HD domains (residues 1–183, highlighted) are 96% identical46. The indicated FLAG-tagged SIX2 fragments were transfected alone into U-2 OS cells and processed for microscopy (middle) or transfected with 5×κB-LUC into HEK293T cells and processed for κB reporter activity (bottom). The highly conserved SD-HD domain was the minimal fragment that inhibited κB reporter activity. This fragment strictly localized to the nucleus, together indicating that SIX-proteins inhibit nuclear activity of NF-κB. Graph data were analyzed as described in Extended Data Fig. 5d. Data are mean±s.d. from 9 independent experiments. c, SIX2 inhibits gene activation from the IL8-promoter. pIL8-LUC plasmid composed of the 1.5 kilobase (kb) promoter region of IL-8 cloned upstream of luciferase gene, was co-transfected with indicated plasmids into HEK293T cells. After 24 hours, cells were treated with mock or 25 ng/ml TNF for 24 hours. The luciferase activity was then measured and analyzed as described in Extended Data Fig. 5d. Data are mean±s.d. from 9 independent experiments. Together, these data suggested that SIX-proteins inhibit NF-κB activation at gene promoters. d, Chromatin immunoprecipitation (ChIP) experiment providing additional evidence that SIX-proteins bind to inflammatory gene promoters as shown in Fig. 3a. Chromatin was prepared from GFP-SIX1 stable cell lines (HCT116 cells) treated with mock or 25 ng/ml TNF for 2 hours. Anti-SIX1 antibodies (or anti-IgG control) were used to immunoprecipitate SIX1 from nuclear extracts. Co-eluted DNA was amplified by primer sets as shown in Fig. 3a. Relative promoter occupancy was normalized to each experimental IgG control. Bars are mean of 2 technical replicates (shown as circles) and data are representative of 3 independent experiments. e, Control experiments corresponding to Fig. 3a and Extended Data Fig. 6f showing GFP-SIX1 expression levels. WT and GFP-SIX1 stable fibroblasts were stimulated with 50 ng/ml TWEAK for 24 hours. GFP-SIX1 expression was measured by western bot. f, SIX1 expression does not affect recruitment of RelA to the IL-8 promoter. Chromatin was prepared from WT or GFP-SIX1 stable fibroblasts and then immunopreciptated with anti-RelA. Bound DNA was amplified and quantified by qPCR. Results were adjusted to “input DNA” that was saved prior to immunopreciptation. Relative enrichment was then normalized to each group’s untreated control. Data are presented as mean±s.d. from 3 independent experiments. g, Control experiments corresponding to Fig. 3c showing that SIX2 inhibits GAL4-RelA and GAL4-RelB induced 5×κB-LUC activity. To validate if GAL4-RelA and GAL4-RelB constructs were functional, GAL4 DNA-binding domain fused RelA or RelB construct was co-transfected with indicated plasmids into HEK293T cells. 48 hours post transfection, the luminescence units were measured. Data are mean±s.d. from 9 (left) and 12 (right) independent experiments. h, Model showing NIK-mediated reactivation of SIX-proteins function in a negative feedback loop to control inflammatory gene expression by targeting gene promoter and inhibiting NF-κB trans-activation function. In quiescent cells (top panel), NIK and SIX-proteins are constitutively ubiquitinated and degraded by the proteasome. Non-canonical NF-κB agonists (e.g. TWEAK, LTα1β2, or BV6) promote degradation of cIAPs, loss of NIK ubiquitination and subsequent NIK protein accumulation (middle panel). Stabilized NIK protein activates non-canonical NF-κB mediated inflammatory gene expression (middle panel). Under conditions of long-term cytokine exposure, NIK-mediated suppression of a currently unknown E3-ubiquitin ligase results in SIX-protein accumulation (bottom panel). Consequently, SIX-proteins suppress inflammatory gene expression by targeting gene promoters and directly inhibiting NF-κB trans-activation function in a negative feedback loop (bottom panel). All western blot and microscopy data are representative of 3 independent experiments. For gel source data, see Supplementary Figure 1.