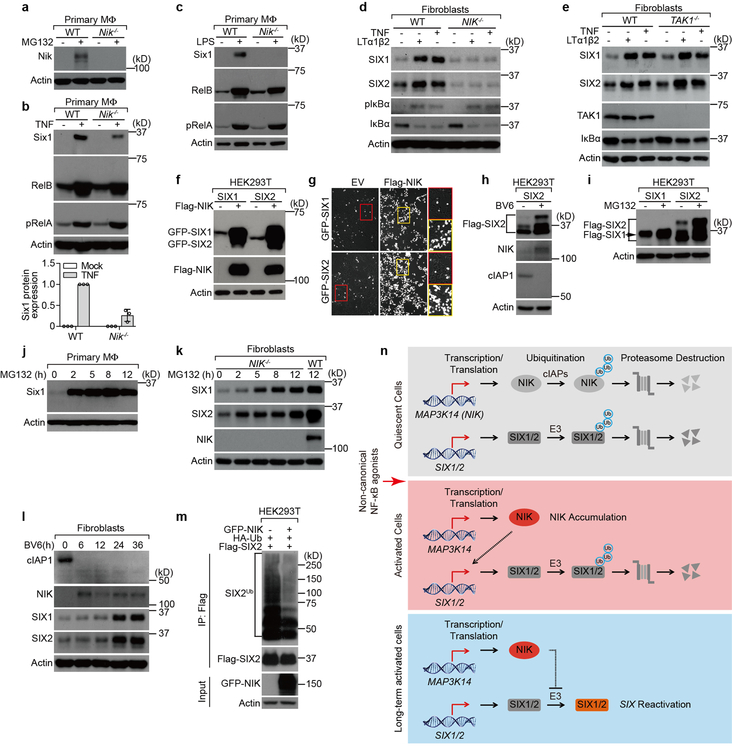
Extended Data Fig. 3. NIK mediates reactivation of SIX-proteins by inhibiting the ubiquitin/proteasome pathway.
a, Control experiment for Fig. 2a, c showing that Nik is expressed in WT BMDMs but not in BMDMs isolated from Map3k14−/− (here forward Nik−/−) mice. As mentioned in Extended Data Fig. 1d, Nik protein is constitutively degraded under quiescent condition. Thus, we employed MG132 proteasome inhibitor to stabilize endogenous Nik protein expression. To validate Nik protein expression, WT and Nik−/− primary BMDM cells were treated with mock or 30 μM MG132 for 12 hours and Nik protein was detected by western blot. b, c, Long term treatment of cells with TNF (b) or LPS45 (c) stabilized Six1 expression through activation of Nik in murine primary BMDM cells. WT and Nik−/− primary BMDMs were treated with 25 ng/ml TNF (b, graph showing quantification of Six1 protein expression in TNF treated cells mean±s.d. from 3 independent experiments as described in Fig. 2c) or 100 ng/ml LPS (c) for 24 hours. d, e, Human SIX1 and SIX2 protein reactivation by long term treatment of cells with both canonical (TNF) and non-canonical (LTα1β2) NF-κB agonists requires NIK, but not TAK1. WT, NIK−/− or TAK1−/− fibroblasts were treated with mock, 25 ng/ml TNF or 50 ng/ml LTα1β2 for 24 hours. LTα1β2 was employed as positive control of a non-canonical NF-κB agonist. TAK1−/− cells were included as control to show TNF and LTα1β2 could induce SIX1 and SIX2 accumulation in a TAK1 independent manner (e). f, g, Ectopic expression of NIK induces expression of recombinant SIX1 and SIX2 driven by the strong CMV promoter in HEK293 cells. Plasmids encoding CMV-driven GFP-SIX1 or GFP-SIX2 were co-transfected into HEK293T cells with empty vector (EV) or Flag-NIK. Western blot (f) and fluorescence microscopy (g) assays were performed to detect expression of GFP-SIX1 and GFP-SIX2 post 48 hours transfection. We estimate that SIX1 and SIX2 protein are expressed in 5–10% of untreated cells, whereas they are expressed in 60–70% of cells when co-transfected with NIK. Microscopy images were taken using a 10× objective (g). h, i, Experiments showing that activation of NIK by BV6 (h) or by inhibition of the proteasome with MG132 (i) stabilizes CMV-Flag-SIX2 expression in HEK293T cells. Flag-SIX2 was transfected into HEK293T cells for 24 hours, cells were then treated with mock or 5 μM BV6 for 24 hours or 30 μM MG132 for 12 hours. j, Inhibition of the 26S proteasome with MG132 induces endogenous Six1 protein expression in primary BMDMs. Cells were treated with 30 μM MG132 for the indicated time. k, Inhibition of the 26S proteasome promotes SIX1 and SIX2 expression in human fibroblasts and this expression occurs in NIK−/− fibroblasts. Experiments were performed as in j. l, Kinetics of cIAP1 degradation and NIK, SIX1 and SIX2 accumulation in human fibroblasts treated with BV6. WT fibroblasts were treated with 5 μM for indicated time. m, NIK potently suppresses SIX2 ubiquitination. HEK293T cells were co-transfected with HA-ubiquitin and Flag-SIX2 along with GFP-NIK as indicated and cells were incubated for 48 hours. SIX2 was immunoprecipitated with anti-Flag antibody. The ubiquitination status of the protein was determined by anti-HA western blot. n, Diagram showing the reactivation mechanism of SIX-proteins in response to non-canonical NF-κB activation. Details are explained in the main text. All data are representative of 3 independent experiments. For gel source data, see Supplementary Figure 1.