Abstract
Background
Biliary tract cancer (BTC) has a high mortality. Primary diagnosis is frequently delayed due to mostly unspecific symptoms, resulting in a high number of advanced cases at the time of diagnosis. Advanced BTCs are in principle chemotherapy sensitive as determined by improved disease control, survival and quality of life (QoL). However, median OS does not exceed 11.7 months with the current standard of care gemcitabine plus cisplatin. Thereby, novel drug formulations like nanoliposomal-irinotecan (nal-IRI) in combination with 5- fluorouracil (5-FU)/leucovorin may have the potential to improve therapeutic outcomes in this disease.
Methods
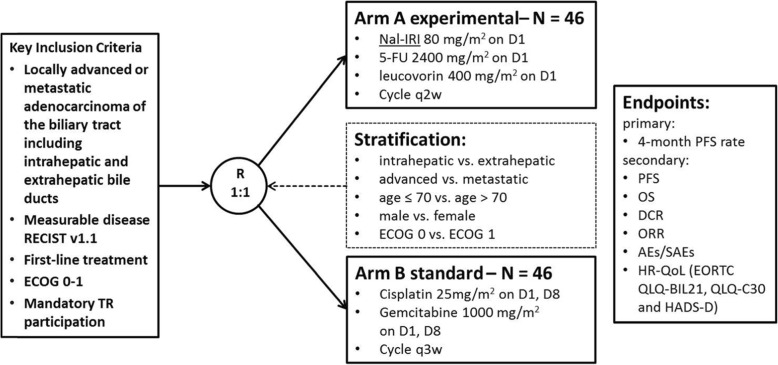
NIFE is an interventional, prospective, randomized, controlled, open label, two-sided phase II study. Within the study, 2 × 46 patients with locally advanced, non-resectable or metastatic BTC are to be enrolled by two stage design of Simon. Data analysis will be done unconnected for both arms. Patients are allocated in two arms: Arm A (experimental intervention) nal-IRI mg/m2, 46 h infusion)/5-FU (2400 mg/m2, 46 h infusion)/leucovorin (400 mg/m2, 0.5 h infusion) d1 on 14 day-cycles; Arm B (standard of care) cisplatin (25 mg/m2, 1 h infusion)/gemcitabine (1000 mg/m2, 0.5 h infusion) d1 and d8 on 21 day-cycles. The randomization (1:1) is stratified for tumor site (intrahepatic vs. extrahepatic biliary tract), disease stage (advanced vs. metastatic), age (≤70 vs. > 70 years), sex (male vs. female) and WHO performance score (ECOG 0 vs. ECOG 1). Primary endpoint of the study is the progression free survival (PFS) rate at 4 months after randomization by an intention-to-treat analysis in each of the groups. Secondary endpoints are the overall PFS rate, the 3-year overall survival rate, the disease control rate after 2 months, safety and patient related outcome with quality of life. The initial assessment of tumor resectability for locally advanced BTCs is planned to be reviewed retrospectively by a central surgical board. Exploratory objectives aim at establishing novel biomarkers and molecular signatures to predict response. The study was initiated January 2018 in Germany.
Discussion
The NIFE trial evaluates the potential of a nanoliposomal-irinotecan/5-FU/leucovorin combination in the first line therapy of advanced BTCs and additionally offers a unique chance for translational research.
Trial registration
Clinicaltrials.gov NCT03044587. Registration Date February 7th 2017.
Keywords: Biliary tract cancer, Cholangiocarcinoma, Chemotherapy, Nanoliposomal-irinotecan, Palliative treatment
Background
Biliary tract cancer (BTC) is a rare type of cancer and ranks beyond 10th in Western World tumor incidence [1]. However, the incidence particularly of intrahepatic BTC is rising, [2, 3] resulting BTC to be the 5th leading cause of cancer related deaths [1]. The main reason for the high mortality of BTCs can be found in the generally advanced stage at primary diagnosis, due to often missing early symptoms [4]. 5-year overall survival rates do not exceed 5% for patients with advanced or metastatic disease [1]. Advanced BTCs respond to chemotherapy, resulting in an improved disease control rate, survival time and quality of life (QoL) [5–7]. However, overall survival rates beyond 10 months remain rare in the palliative setting. The current standard of care combines conventional chemotherapeutic agents for patients who are in a good performance status. Therapy is based on the ABC-02 phase III trial that demonstrated a beneficial progression-free (PFS) and overall survival (OS) for a combination of gemcitabine plus cisplatin compared to gemcitabine alone (Cis + Gem vs. Gem: OS 11.7 vs. 8.1 months; PFS 8.0 vs. 5.0 months) [6]. However, the therapeutic landscape in oncology is steadily evolving bringing novel compounds into daily clinical routine in various cancer entities. Several antibodies and inhibitors like cetuximab or sorafenib were evaluated in advanced BTC, but failed to improve outcome [5, 8]. Irinotecan combined with 5-FU showed promising results in the 1st- [9] and 2nd-line treatment [10] of advanced BTC and is commonly used as therapeutic option after failure of the 1st-line therapy with gemcitabine/cisplatin. Consequently, encapsulation of irinotecan in pegylated liposomes could be of value in advanced BTC as efficacy and tolerability of this drug are already proven in a number of solid tumors including pancreatic [11], gastric [12] and colorectal cancers [13]. Nanoliposomal-irinotecan (nal-IRI) showed extended plasma half-life and increased intratumoral concentrations compared to conventional irinotecan in preclinical models [14–16]. The NAPOLI-1 trial transferred this to the patient and demonstrated in a phase III setting a significantly prolonged OS for 2nd-line therapy with nal-IRI/5-fluorouracil (5-FU)/leucovorin (LV) in patients with metastatic pancreatic cancer compared to 5-FU/LV only [11]. The superiority shown in the NAPOLI-1 trial provides compelling evidence for a potential efficacy in advanced BTC. The toxicity profile of nal-IRI is similar to what has been described for irinotecan that is routinely used in clinical practice by oncologists [12].
The NIFE phase II trial aims to challenge the current palliative first-line therapy of BTC by use of nanoliposomal-irinotecan/5-FU/leucovorin and to further establish specific biomarker signatures.
Methods and study design
NIFE is an interventional, prospective, randomized, controlled, open label, two-sided phase II study, using the optimal two-stage design of Simon in each of the experimental arms.
Study objectives
Primary objective
PFS rate at 4 months, defined as the proportion of patients with non-progressive disease 4 months after randomization by intention-to-treat analysis
Secondary objectives:
Overall progression-free survival
3-years overall survival
Disease control rate according to RECIST 1.1 [17] after 2 months
Objective tumor response rate (ORR) according to RECIST 1.1 [17]
Toxicity/safety according to CTCAE-criteria version 4.03 (≥ Grade 3/4)
Patient-related outcome/quality of life/time to definitive deterioration (TUDD) to be assessed with the following tools: EORTC QLQ-BIL21, QLQ-C30 and HADS-D
Tumor resectability in accordance with a retrospective central surgical board compared to local surgical review
Radiological response according to RECIST 1.1 [17] and volumetry determined by a retrospective central radiological review
Exploratory objectives:
Exploratory biomarkers analysis (cfDNA exome sequencing, transcriptome, miRNA-arrays prior to and after start of treatment, and on progression).
Establishment of predictive/prognostic biomarker profiles for advanced BTC
Tumor evolution under chemotherapy
Patient selection and randomization
Approximately 120 patients have to be screened to get 92 randomized patients (46 patients per arm). In the study, 30 participating centers are planned. The trial is randomized in a 1:1 ratio to the experimental (Arm A) or standard arm (Arm B) to get comparable sample sizes by stratified permutated block randomization to avoid a selection bias, see Fig. 1. The randomization (1:1) is stratified for tumor site (intrahepatic vs. extrahepatic biliary tract), disease stage (advanced vs. metastatic), age (≤70 vs. > 70 years) [18], sex (male vs. female) and WHO performance score (ECOG 0 vs. ECOG 1).
Fig. 1.
Flow diagram NIFE trial
Main inclusion and exclusion criteria
Inclusion criteria:
Histologically confirmed, non-resectable, locally advanced or metastatic adenocarcinoma of the intrahepatic or extrahepatic biliary tract (not papillary cancer or gallbladder cancer)
Non-resectability has to be stated by the local multidisciplinary tumor board
Measurable or assessable disease according to RECIST 1.1 [17]
ECOG performance status 0–1
Age ≥ 18 years at time of study entry
Life expectancy of more than 3 months
If applicable, adequately treated biliary tract obstruction before study entry with total bilirubin concentration ≤ 2 x ULN
- Adequate blood count, liver-enzymes, and renal function:
- ◦ AST (SGOT)/ALT (SGPT) ≤ 5 x institutional upper limit of normal
- ◦ Serum Creatinine ≤1.5 x institutional ULN and a calculated glomerular filtration rate ≥ 30 mL per minute
- ◦ Patients not receiving therapeutic anticoagulation must have an INR < 1.5 ULN and PTT < 1.5 ULN within 7 days prior to randomization
No prior palliative chemotherapy for biliary tract cancer
No adjuvant treatment within 6 months prior to study entry
Written informed consent including participation in translational research
Exclusion criteria:
Clinically significant cardiovascular disease (incl. Myocardial infarction, unstable angina, symptomatic congestive heart failure, serious uncontrolled cardiac arrhythmia) within 6 months before enrollment
Prior (< 3 years) or concurrent malignancy (other than biliary-tract cancer) which either progresses or requires active treatment. Exceptions are: basal cell cancer of the skin, pre-invasive cancer of the cervix, T1a or T1b prostate carcinoma, or superficial urinary bladder tumor [Ta, Tis and T1].
Known Gilbert-Meulengracht syndrome
Known chronic hypoacusis, tinnitus or vertigo
Previous enrollment or randomization in the present study (does not include screening failure).
Staging assessments
Medical history and demographics including dates and description of initial diagnosis of advanced biliary tract cancer and relevant concurrent illness
Complete physical examination including: weight, height, BSA, vital signs (blood pressure, heart rate, respiratory rate and oral body temperature)
Residual symptoms/toxicities from previous therapies should be recorded according to the NCI Common Toxicity Criteria
ECOG Performance Status
Review of prior/concomitant medications
Tumor assessment according to RECIST 1.1 [17] done by local investigator in the context of standard care (contrast enhanced multislice CT of the abdomen or abdominal MRI and an enhanced multislice thoracic CT scan)
EORTC QLQ-BIL21, QLQ-C30 and HADS-D questionnaire
Nutritional risk score
12-lead ECG
Hematological tests, Clinical chemistry
Serum Tumor Marker (Ca 19–9, CEA)
Treatment
Treatment is planned in an outpatient setting for all study drugs and will continue until there is evidence of disease progression or occurrence of any other discontinuation criterion. If nal-IRI or cisplatin have to be discontinued permanently under therapy for a reason other than progressive disease, treatment should continue with the remaining drug in the trial, with full adherence to all protocol-related requirements. Within a therapy cycle, treatment should continue on schedule, but a variance of ±5 days may be allowed to accommodate holidays, weekends or other justifiable events.
Arm A (experimental arm):
Nanoliposomal-irinotecan 80 mg/m2 as 1.5 h infusion
5-fluorouracil 2400 mg/m2 as 46 h infusion
Leucovorin 400 mg/m2 as 0.5 h infusion
Cycle q2w ± 5 days
Arm B (standard arm):
Cisplatin 25 mg/m2 as 1 h infusion on day 1 and day 8
Gemcitabine 1000 mg/m2 as 0.5 h infusion on day 1 and day 8
Cycle q3w ± 5 days
Follow-up
All subjects undergo follow-up for survival until the end of the study irrespective of subsequent treatments, or until the sponsor ends the study (follow-up extension phase). Patient contact is to be established by telephone interview or face-to-face, whichever prevails.
The following procedures will be performed during follow-up every 8 weeks:
Assessment of survival status
Anti-cancer treatments must be recorded during follow up
Reporting of all adverse events (AEs) and severe adverse events (SAEs) within 4 weeks after the end of treatment (EoT) visit
Sample size calculation and statistical analysis
Simon’s optimal two-stage design was used for sample size calculation for each group by OneArmPhaseTwoStudy software [19]. H0: less than 40% of patients are progression-free by 4 months of nal-IRI plus 5-FU/leucovorin. Alternative hypothesis: ≥60% of patients are progression-free by 4 months of nal-IRI plus 5-FU/leucovorin. If 7 or less of the first 18 patients assigned to nal-IRI plus 5-FU/leucovorin have a tumor response or stable disease at 4 months, H0 will be accepted and the study will be terminated. If 8 or more patients with tumor response or stable disease are observed, another 28 patients in each treatment group are to be included. At the final analysis, H0 will be accepted if less than 23 of the total 46 patients in the nal-IRI plus 5-FU/leucovorin group had a tumor response or stable disease at 4 months. With this design, alpha = 10% (significance level) and power = 90%. As the study will be analyzed as intention-to-treat analysis (ITT), all patients will be analyzed (missing data will be considered as failure). Hence, a sample size of n = 46 per treatment arm and a total N = 92 enrolled and randomized patients is required. It is assumed that approx. 120 patients need to be screened for eligibility.
Quality of life assessment and time to definitive deterioration
Health related quality of life (HRQL) will be assessed by using the EORTC QLQ-C30 questionnaire version 3.0. The questionnaire contains 5 functions (physical, role, cognitive, emotional, and social), 9 symptoms (fatigue, pain, nausea and vomiting, dyspnea, loss of appetite, insomnia, constipation, diarrhea and financial difficulties) and the global health status/quality of life (GBH/QoL) [20]. To further specify the assessment the module for biliary tract cancer (QLQ-BIL21) with 21 items related to disease symptoms, treatment side effects and emotional issues in BTC is included [21]. A calculation of the median time to definitive deterioration (TUDD) using the EORTC QLQ-C30 questionnaire data is planned. The TUDD will be calculated in accordance to Anota et al. and Bonnetain et al. and is defined as an ongoing deterioration of at least 5 points compared to the baseline [22, 23]. The emotional and social impact of being diagnosed with BTC is highly relevant. To detect anxiety and depression, which are the most common co-morbidities of physical illness, the HADS-D questionnaire (Hospital Anxiety and Depression Scale – German version) is used. The HADS-D has 14 items (7 anxiety, 7 depression) each with a 4-point verbal rating scale scored from 0 to 3. The scale deliberately avoids physical indicators of mental disorders (e.g., insomnia, weight loss) and severe psychopathological symptoms allowing high sensitivity with proven psychometric quality criteria [24, 25].
HRQL should be assessed at following time points:
At baseline, within 7 days prior to randomization
Before the beginning of each cycle of systemic therapy
At end of treatment visit
Quality of life assessment should be performed even when chemotherapy cannot be given at the beginning of a cycle e.g. due to toxicity reasons.
Nutritional screening
The nutritional risk score (NRS) questionnaire will be used for the evaluation of nutritional anomalies. Malnutrition and weight loss are common problems in advanced BTC patients and contribute to morbidity and mortality. Furthermore, tolerance of chemotherapy is often worse in patients with severe malnutrition. The NRS questionnaire is a simple tool to screen patients for malnutrition [26]. The questionnaires will be completed at time of screening, every 8 weeks under therapy and at the EoT visit.
Translational research
This trial provides the opportunity to systematically obtain biologic material from therapy naive patients suffering from advanced BTC for comprehensive molecular characterization. It allows to assess treatment associated tumor evolution under 1st-line palliative chemotherapy with different regimens. Consequently, we will collect tissue samples obtained for initial diagnosis for exome sequencing best versus worst responders. We hypothesize that exome sequencing of microdissected tumor cells from initially taken core biopsies will identify important biologic differences between tumors responding to cytotoxic chemotherapy compared to those not responding to the treatment and thereby provide potential predictive markers. In parallel, blood samples of each patient will be taken prior to treatment, after 4–5 weeks of treatment, thereafter in parallel to radiologic tumor assessments until disease progression (radiologically confirmed). Circulating cell-free tumor DNA will be extracted and analyzed by targeted genotyping in order to verify the potential of liquid biopsy as a disease diagnosis and treatment monitoring tool, as previously shown. Mutation profiles obtained from tissue and blood will be compared to evaluate whether tumor DNA analysis from blood yields a pattern comparable to tumor tissue and could be used to establish “easy to obtain” prognostic and predictive markers for nal-IRI based treatment.
Ethical aspects, trial registration
All patients have to sign written informed consent including participation in translational research and any locally-required authorization (including EU Data Privacy Directive in the EU, Declaration of Helsinki) obtained from the subject prior to performing any protocol-related procedures, including screening evaluations. The ethics committee of Ulm University approved the NIFE-trial as leading ethics committee for all German sites according to German regulative laws for trials (Arzneimittelgesetz). In addition, local ethics committees approved the participating sites. The trial is registered with ClinicalTrials.gov (NCT0344587).
Discussion
Median overall survival in patients with advanced BTC is still devastating, generally not exceeding 1 year with the current therapeutic concepts. The results of the ABC-02 6 and the BINGO trial [5] defined gemcitabine/cisplatin (or oxaliplatin) as treatment of choice in advanced BTC first line therapy. Therefore the investigators reported a progression-free survival (PFS) rate of 54% at 4-months in the gemcitabine/oxaliplatin group. Irinotecan was evaluated in several combinations in advanced BTC as first-line treatment, [27–30] showing the most promising results in combination with a thymidylate synthase inhibitor [31–33]. There is evidence that the nanoliposomal formulation of irinotecan may confer improved efficacy of the drug [14, 15, 34–37]. This encouraged us to try nal-IRI/5-FU/leucovorin in the first line treatment of advanced BTC, particularly given the positive data on safety and tolerability in both phase II and III trials as well as in real-life data in PDAC [11, 38, 39]. The NIFE trial aims to update and widen the treatment landscape in advanced BTC by using Nal-IRI/5-FU/leucovorin. For the NIFE trial we assume that ≥60% of patients are progression-free after 4 months of nal-IRI/5-FU/leucovorin. An interim analysis is planned after 18 patients have been enrolled to confirm the hypothesis.
The knowledge on BTC biology is still limited compared to other solid cancers. Recent sequencing studies shed more light on the mutational landscape of BTC and encourage the use of novel therapeutic targets [40–42]. However, a synergistic chemotherapy backbone is commonly needed like in other difficult to treat GI malignancies [43, 44]. Thereby, advanced BTC already showed the limitations of such strategies with no effects by adding cetuximab to standard chemotherapy in the BINGO trial [5]. Anyhow the spectrum of BTC mutations appears to lie within other gastrointestinal epithelial cancers with similar oncogenic mutations [42, 45, 46]. As a consequence a proper definition of BTC subtypes is paramount potentially guiding future treatment approaches. Therefore an expanded liquid biopsy program like that included in the NIFE trial may allow new insights on stratification and especially on the development of the mutational landscape under therapy.
To sum up, the NIFE trial evaluates the potential of nanoliposomal-irinotecan/5-FU/leucovorin in the first line therapy of advanced BTCs and additionally offers a unique chance for translational research.
Acknowledgements
The phase 2 trial started in 01/2018 and is still ongoing and recruiting patients. Further study information is open-access available at clinicaltrials.gov (NCT03044587). Outlines of the protocol were presented as a poster at ASCO 2018 and ESMO 2018.
Abbreviations
- (m)DFS
(median) disease free survival
- (m)OS
(median) overall survival
- (m)PFS
(median) progression free survival
- 5-FU
5- fluorouracil
- AE
Adverse event
- AIO
Arbeitsgemeinschaft Internistische Onkologie
- BSA
Body surface area
- BTC
Biliary tract cancer
- CBC
Complete blood count
- cfDNA
Circulating free DNA
- Cis
Cisplatin
- CT
Computed Tomography
- CTC
Common toxicity criteria
- CTCAE
Common Terminology Criteria for Adverse Events
- DCR
Disease control rate
- ECG
Electrocardiogram
- ECOG
Eastern Cooperative Oncology Group
- EORTC
European Organization for Research and Treatment of Cancer
- EOT
End of treatmend
- FOLFIRINOX
Fluorouracil leucovorine, irinotecan, oxaliplatin
- GBH
Global health status
- G-CSF
Granulocyte-colony stimulating factor
- Gem
Gemcitabine
- HADS-D
Hospital Anxiety and Depression Scale
- HR
Hazard ratio
- HRQL
Health related quality of life
- ITT
Intention to treat
- LV
Leucovorin
- MRI
Magnetic Resonance Imaging
- Nab-Paclitaxel
Nano albumin bound Paclitaxel
- Nal-IRI
Nanoliposomal-Irinotecan
- NCI
National Cancer Institute
- NRS
Nutritional risk score
- ORR
Objective response rate
- PDAC
Pancreatic ductal adenocarcinoma
- QLQ-C30
Quality of life questionnaire-core 30
- QoL
Quality of life
- RECIST
Response Evaluation Criteria in Solid Tumors
- SAE
Severe adverse event
- TUDD
Time until definitive deterioration
- ULN
Upper limit of normal
- WHO
World health organization
Authors’ contributions
TJE, LP wrote the protocol. RM conducted statistical trial planning. TJE and AWB handled ethics and regulatory affairs. TJE and LP wrote the paper draft. AWB, AKB, GTO, EG, SA, LFvW, TS contributed in the trial design and modifications and data collection. All authors have approved the final version of the manuscript.
Funding
The trial is funded by an unrestricted grant by Servier. The study has not undergone peer-review by the funding body. Data collection, analysis, interpretation of data and manuscript writing are independent from Servier. The protocol hasn’t undergone peer-review by the funding body.
Availability of data and materials
Not applicable. Data sharing is planned once the trial is completed.
Ethics approval and consent to participate
The ethics committee of Ulm University (Ethikkommission der Universität Ulm) approved the NIFE-trial as leading ethics committee for all German sites (reference number 11/17). In addition, local ethics committees approved the participating sites. The local ethics committees are as follows: Ethikkommission bei der Sächsischen Landesärztekammer, Ethikkommission der Bayerischen Landesärztekammer, Ethikkommission des Fachbereichs Medizin der Philipps-Universität Marburg, Ethikkommission der Landesärztekammer Baden-Württemberg, Ethikkommission der Medizinischen Fakultät der HHU Düsseldorf, Ethikkommission des Fachbereichs Medizin der Goethe-Universität Frankfurt am Main, Ethikkommission der Ärztekammer Mecklenburg-Vorpommern, Ethikkommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, Ethikkommission der Ärztekammer Nordrhein, Geschäftsstelle der Ethikkommission des Landes Berlin, Ethikkommission der Medizinischen Fakultät der Universität Duisburg-Essen Universitätsklinikum Essen, Ethikkommission des Landes Sachsen-Anhalt c/o Landesamt für Verbraucherschutz, Ethikkommission der FSU Jena, Ethikkommission bei der Ärztekammer Niedersachsen, Ethikkommission der Medizinischen Fakultät der Universität zu Köln, Ethikkommission bei der Landesärztekammer Hessen. The trial is registered at Clinicaltrials.gov (NCT03044587) and the European Clinical Trials Database (2016–002467-34). All patients signed informed consent according to GCP.
Consent for publication
Not applicable.
Competing interests
Nanoliposomal-Irinotecan, 5-FU and leucovorin are provided by Servier. LP and TJE received travel grants from IPSEN Pharma, the other authors declare no conflicts of interest. The trial is sponsored according to German regulatory laws by the AIO Studien GmbH. The study was not externally reviewed.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
L. Perkhofer, Email: lukas.perkhofer@uniklinik-ulm.de
A. W. Berger, Email: andreas.berger@vivantes.de
A. K. Beutel, Email: alica.beutel@uniklinik-ulm.de
E. Gallmeier, Email: eike.gallmeier@med.uni-marburg.de
S. Angermeier, Email: stefan.angermeier@kliniken-lb.de
L. Fischer von Weikersthal, Email: weikersthal.ludwig@gesundheitszentrum.klinikum-amberg.de
T. O. Goetze, Email: Goetze.Thorsten@khnw.de
R. Muche, Email: rainer.muche@uni-ulm.de
T. Seufferlein, Email: thomas.seufferlein@uniklinik-ulm.de
T. J. Ettrich, Email: thomas.ettrich@uniklinik-ulm.de
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.von Hahn T, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46:1092–1098. doi: 10.3109/00365521.2011.589472. [DOI] [PubMed] [Google Scholar]
- 3.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel A, Wege H, Caca K, Nashan B, Neumann U. The diagnosis and treatment of cholangiocarcinoma. Dtsch Arztebl Int. 2014;111:748–754. doi: 10.3238/arztebl.2014.0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malka D, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–828. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moehler M, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125–3135. doi: 10.1016/j.ejca.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Feisthammel J, et al. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol. 2007;30:319–324. doi: 10.1097/01.coc.0000258124.72884.7a. [DOI] [PubMed] [Google Scholar]
- 10.Guion-Dusserre JF, Lorgis V, Vincent J, Bengrine L, Ghiringhelli F. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:2096–2101. doi: 10.3748/wjg.v21.i7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang-Gillam A, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 12.Roy AC, et al. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol. 2013;24:1567–1573. doi: 10.1093/annonc/mdt002. [DOI] [PubMed] [Google Scholar]
- 13.Chibaudel B, et al. PEPCOL: a GERCOR randomized phase II study of nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with leucovorin/5-fluorouracil as second-line therapy in metastatic colorectal cancer. Cancer Med. 2016;5:676–683. doi: 10.1002/cam4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond DC, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 15.Kalra AV, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74:7003–7013. doi: 10.1158/0008-5472.CAN-14-0572. [DOI] [PubMed] [Google Scholar]
- 16.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 17.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.McNamara MG, et al. Systemic therapy in younger and elderly patients with advanced biliary cancer: sub-analysis of ABC-02 and twelve other prospective trials. BMC Cancer. 2017;17:262. doi: 10.1186/s12885-017-3266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kieser M, Wirths M, Englert S, Kunz CU, Rauch G. OneArmPhaseTwoStudy: an R package for planning, conducting, and Analysing single-arm phase II studies. 2017. p. 28. [Google Scholar]
- 20.Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 21.Friend E, et al. Development of a questionnaire (EORTC module) to measure quality of life in patients with cholangiocarcinoma and gallbladder cancer, the EORTC QLQ-BIL21. Br J Cancer. 2011;104:587–592. doi: 10.1038/sj.bjc.6606086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anota A, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5–18. doi: 10.1007/s11136-013-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnetain F, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010;46:2753–2762. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Herrmann C, Scholz KH, Kreuzer H. Psychologic screening of patients of a cardiologic acute care clinic with the German version of the hospital anxiety and depression scale. Psychother Psychosom Med Psychol. 1991;41:83–92. [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Reilly HM, Martineau JK, Moran A, Kennedy H. Nutritional screening--evaluation and implementation of a simple nutrition risk score. Clin Nutr. 1995;14:269–273. doi: 10.1016/S0261-5614(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 27.Karachaliou N, et al. A multicenter phase II trial with irinotecan plus oxaliplatin as first-line treatment for inoperable/metastatic cancer of the biliary tract. Oncology. 2010;78:356–360. doi: 10.1159/000320462. [DOI] [PubMed] [Google Scholar]
- 28.Sohal DP, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol. 2013;24:3061–3065. doi: 10.1093/annonc/mdt416. [DOI] [PubMed] [Google Scholar]
- 29.Chung MJ, et al. Prospective phase II trial of gemcitabine in combination with irinotecan as first-line chemotherapy in patients with advanced biliary tract cancer. Chemotherapy. 2011;57:236–243. doi: 10.1159/000328021. [DOI] [PubMed] [Google Scholar]
- 30.Yoo C, et al. Multicenter phase II study of Oxaliplatin, irinotecan, and S-1 as first-line treatment for patients with recurrent or metastatic biliary tract Cancer. Cancer Res Treat. 2018;50:1324–1330. doi: 10.4143/crt.2017.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, et al. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br J Cancer. 2018;119:291–295. doi: 10.1038/s41416-018-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kus T, Aktas G, Kalender ME, Sevinc A, Camci C. Comparison of FOLFIRINOX chemotherapy with other regimens in patients with biliary tract cancers: a retrospective study. J Gastrointest Cancer. 2017;48:170–175. doi: 10.1007/s12029-016-9880-y. [DOI] [PubMed] [Google Scholar]
- 33.Belkouz A, et al. Efficacy and safety of FOLFIRINOX in advanced biliary tract cancer after failure of gemcitabine plus cisplatin: a phase II trial. J Clin Oncol. 2019;37:4086. doi: 10.1200/JCO.2019.37.15_suppl.4086. [DOI] [Google Scholar]
- 34.Noble CO, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 35.Kang MH, et al. Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in Ewing's family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression. Clin Cancer Res. 2015;21:1139–1150. doi: 10.1158/1078-0432.CCR-14-1882. [DOI] [PubMed] [Google Scholar]
- 36.Neuzillet C, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol. 2012;18:4533–4541. doi: 10.3748/wjg.v18.i33.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen AK, et al. Influence of liposomal irinotecan (nal-IRI) and non-liposomal irinotecan, alone and in combination, on tumor growth and angiogenesis in colorectal cancer (CRC) models. J Clin Oncol. 2018;36:711. doi: 10.1200/JCO.2018.36.4_suppl.711. [DOI] [Google Scholar]
- 38.Passero FC, Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev Anticancer Ther. 2016;16:697–703. doi: 10.1080/14737140.2016.1192471. [DOI] [PubMed] [Google Scholar]
- 39.Glassman DC, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer. 2018;18:693. doi: 10.1186/s12885-018-4605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardell CP, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959–969. doi: 10.1016/j.jhep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Ahn DH, et al. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer. 2016;122:3657–3666. doi: 10.1002/cncr.30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura H, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 44.Wilke H, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 45.Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 46.Zill OA, et al. The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced Cancer patients. Clin Cancer Res. 2018;24:3528–3538. doi: 10.1158/1078-0432.CCR-17-3837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. Data sharing is planned once the trial is completed.