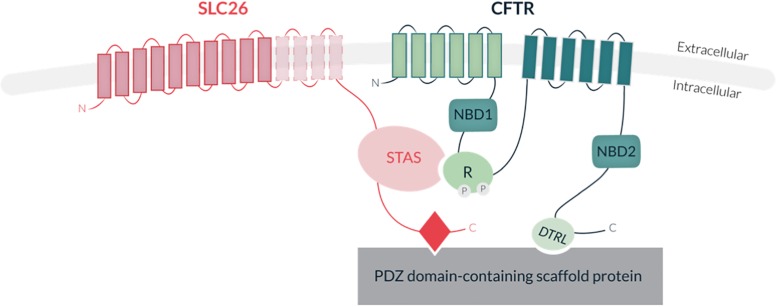
FIGURE 5.
Schematic representation of SLC26 protein structure and interaction with the Cystic Fibrosis Transmembrane conductance Channel (CFTR). SLC26 proteins share a conserved transmembrane region of 10–14 hydrohobic spans, associated with their anion transport activity, and a cytoplasmic STAS domain (Sulfate Transporter and Anti-Sigma factor antagonist), involved in protein-protein interaction and regulation. Some members also contain a PDZ binding motif at their carboxy-terminal extremity. The CFTR protein consists of two transmembrane domains (TMD) (each containing six spans of alpha helices), two nucleotide-binding domains (NBD1 and NBD2) and a central regulatory domain (R-domain). CFTR activity is regulated by PKA-phosphorylation of the R-domain and ATP binding and hydrolysis at the two NBDs. Direct interaction of SLC26 with CFTR is mediated by the STAS domain and the regulatory (R) domain of CFTR. Indirect interaction of the proteins occurs through binding of both SLC26s and CFTR to common PDZ motif-containing scaffold proteins.