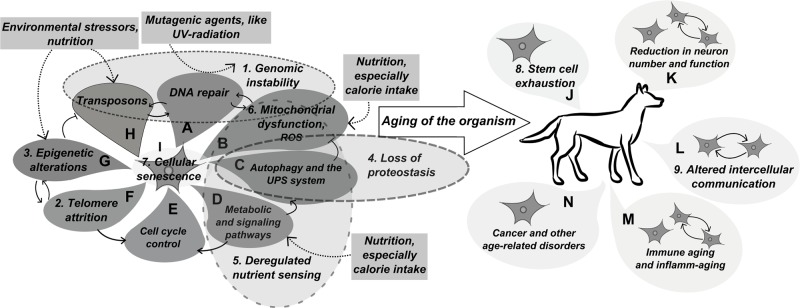
Figure 1.
Main mechanisms of aging.The figure depicts the mechanism that underlie cellular aging and, consequently, aging of a multicellular organism. The main mechanisms defined as hallmarks of aging by López-Otín et al. in 2013 are numbered according to their order in the main text. (A) The DNA repair machinery provides the first line of defence against mutagenic agents and also interacts with mobile DNA elements through repairing double strand breaks induced by transposons. Impaired function of this machinery can be a main contributor to Genomic instability (1). (B) Reactive oxygen species produced by mitochondrial respiration represent an inner source for DNA lesions, thus interact with the DNA repair machinery: proper functionality of DNA repair enzymes is required to protect cells from oxidative damage, however elevation in the level of oxidative stress may overburden the repair machinery. Age-related Mitochondrial dysfunction (6) can lead to increased oxidative and apoptotic burden. In addition, increased oxidative stress can also affect proteostasis. (C) Macroautophagy is a protective mechanism against malfunctioning mitochondria that might cause oxidative stress, while together with other clearance mechanisms it also functions to remove misfolded proteins and protein aggregates. It also functions as a mechanism for programmed cell death (indicated with dashed line between autophagy and cell cycle control). Together with the ubiquitin-proteasome system (not indicated separately on the figure) its malfunction may lead to Loss of proteostasis (4) in cells. (D) The activity of the autophagy machinery is strictly regulated by different signalling pathways, many of which function in metabolite sensing and cell growth control. Dysruption in these signaling pathways is summarized as Deregeulated nutrient sensing (5) by López-Otín et al (2013). (E) Cell cycle control is a main determinant of Cellular senescence (7). Also, on a multicellular level, dysregulation of cell cycle control may decrease lifespan by initiating tumour formation. (F) In many species telomeres function as a measuring mechanisms to limit the number of potential cell cycles. When telomere length reaches a critical shortness, it will activate cell cycle control mechanisms to render the cell into a senescent state. The telomerase enzyme was also shown to interact with global genomic chromatin maintenance. In general, Telomere attrition (2) is a main hallmark of aging in most mammalian species. (G) Epigenetic regulation involves two main mechanisms, the methylation of CpG islands and modification of the chromatin structure through histone proteins. Chromatin structure at telomeres is important for telomere maintenance and the repression of retroelements by CpG methylation may prevent DNA damage caused by transposon mobilisation. Epigenetic alterations (3) are also linked to aging in both humans and model organisms. (H) Derepression of mobile DNA elements, primarily of retroelements in mammalian genomes, may result in an increased frequency of double strand breaks and insertion mutagenesis leading to increased Genomic instability (1). (I) Altogether, the molecular mechanisms of aging eventually result in Cellular senescence (7). (J) Cellular senescence will lead to reduced function of tissues in a multicellular organism. Depletion of tissue renewing stem cells (8) is also a main hallmark of organismal aging and is a result of cellular senescence and increased activation / differentiation of dormant stem cells in certain tissues. (K) Loss of stem cells and cellular senescene will lead to functional decline in the central nervous system. (L) Altered intercellular communication (9) is also considered a main hallmark of aging in multicellular organisms, as coordinated activity of cells is essential for proper tissue functionality. (M) The immune system can be affected considerably by altered intercellular communication, and this could also lead to increased inflammation in the body, called inflamm-aging. The elevated levels of inflammation may also result from the increasing number of senescent / apoptotic cells. (N) Together with intracellular changes, mainly loss of genomic integrity, disrupted proteostasis and signalling pathways, the imbalanced function of the immune system will contribute to the occurrence of age-related diseases.