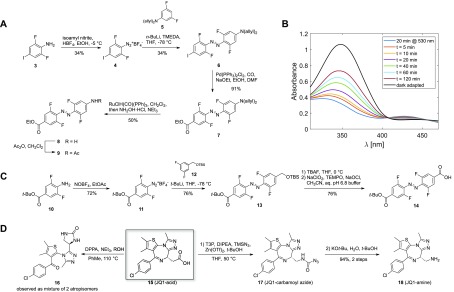
Figure 2.
(A) Synthetic approach toward unsymmetrical amino acid azobenzene linker 8. (B) Bistability measurement of model substrate 9, starting with enriched cis-9 (t = 0) after a 20 min irradiation at 530 nm (50 μM in CH3CN). (C) Synthetic approach toward monoprotected diacid azobenzene building block 14. (D) Classical Curtius conditions mainly generated urea 16 under reflux conditions, preventing access to 18. Milder Curtius conditions under Lewis-acid catalysis allowed isolation of 17 which could be transformed into JQ-1 amine 18.