Abstract
Aims:
The purposes of this study were to determine the anticancer activity of Xestospongia testudinaria sponge isolate and identify the responsible compounds.
Materials and Methods:
The metabolites were extracted using methanol maceration at room temperature. The separation and purification of metabolites were performed using fractionation and column chromatography. The toxicity was examined using the brine shrimp lethality assay, and the toxic isolates were tested for anticancer activity against HeLa cells. Gas chromatography-mass spectrometry analysis was used to identify the compounds in the isolate.
Results:
When the methanol extract was partitioned with n-hexane, chloroform, and n-butanol, the chloroform fraction was the most toxic, with a concentration that results in 50% lethality (LC50) value of 39.81 ppm. After separation of the chloroform extract, fraction B (FB) was the most toxic, with an LC50 value of 44.67 ppm. The isolate from FB showed anticancer activity with a concentration at which 50% of growth was inhibited (IC50) of 2.273 ppm. In total, 21 compounds were identified in anticancer isolates: Nonanedioic acid; tetradecanoic acid; trans-phytol; 2-pentadecanone-6,10,14-trimethyl; pentadecanoic acid; 2-hexadecen-1-ol, 3,7,11,15-tetramethyl-; pentadecanoic acid; 2-hexadecen-1-ol, 3,7,11,15-tetramethyl-; 2,3,7-trimethyloctanal; palmitic acid; docosanoic acid, ethyl ester; 1,E-11,Z-13-octadecatriene; chloromethyl 4-chlorododecanoate; 1-tricosene; 9,12-octadecadienoic acid; 4,8,12,16-tetramethylheptadecan-4-olide; 1-docosene; heneicosane; phosphonic acid, dioctadecyl ester; dodecane,4,6-dimethyl-; n-tetratriacontane; 1-iodohexadecane; and n-heneicosane.
Conclusion:
These findings indicate that the isolate of X. testudinaria can be used as a natural anticancer toward HeLa cell.
Keywords: anticancer activity, HeLa cell, Xestospongia testudinaria
Introduction
Cancer is caused by the abnormal growth and development of cells in the body [1]. During the course of the disease, these cells may spread to other parts of the body, ultimately resulting in death where the currently available therapies are not effective [2-5]. Cancer is the second most common cause of death after heart disease, responsible for more than 500,000 deaths per year in the United States [6] and an estimated 100 new patients in every 100,000 inhabitants diagnosed every year in Indonesia [7]. Current cancer treatments generally combine surgical and radiation methods with chemotherapy [8], but the methods have not led to optimal results.
The development of new anticancer drugs has become a priority because of the high cost and low selectivity of the currently available drugs [9]. A wide range of natural resources, including marine organisms, has been considered in the search for new anticancer compounds [10]. Some researchers believe that sponges are a potential source of bioactive compounds from the ocean. Nearly 5000 compounds have been isolated from these sponges and determined to have various biological effects such as antimicrobial, antifungal, antiviral, and anticancer activities [11]. Metabolite extracts from sea sponges contain bioactive compounds with antiviral [12], anti-HIV, anti-inflammatory, antifungal, antileukemia [13], enzyme inhibitory [14], antimalarial [15], antioxidant [16], cytoprotective, and antitumor [17] activity.
Few studies have assessed the anticancer activity of Xestospongia testudinaria sponge. The alcohol and n-hexane extracts (EH) from X. testudinaria from the Saudi Red Sea were found to have strong cytotoxic activity against human cervical cancer (HeLa), human hepatocellular carcinoma (HepG-2), and human medulloblastoma (Daoy) cell lines [18]. The methanol extract of X. testudinaria sponge from Sanur, Bali, Indonesia had anticancer activity against HeLa cells with an IC50 value of 1327 ppm [19]. The antitumor activity of two new polyacetylene sponges Xestospongia spp. from the Red Sea was also reported [20]. Moreover, effects other than anticancer of X. testudinaria sponge extract have been widely reported: X. testudinaria sponge showed antibacterial activity [20-22]; Vietnamese X. testudinaria sponge has antifouling activity of 26,27-cyclosterols [23]; the toxicity of the methanol extract of X. testudinaria sponge was reported to have a concentration that results in 50% lethality (LC50) value of 31.62 ppm [24,25]. Five compounds identified in the sponges (sapinofuranone, xestospongic acid, 24-hydroperoxy-24-vinyl-cholesterol, saringosterol, and 29-hydroperoxystigmasta-5,24-dien-3β-ol) were toxic to Artemia salina larvae with LC50 values between 0.56 and 6.99 μM [24].
A preliminary test for anticancer activity was conducted using the brine shrimp lethality assay [26]. If material has an LC50 value of below 1000 ppm, this indicates anticancer potential and suggests the use of further anticancer testing in HeLa cells [19,27]. The methanol extract of X. testudinaria sponge from Sanur Bali had anticancer activity with a concentration at which 50% of growth was inhibited (IC50) of 1327 ppm [19].
The purposes of this study were to determine the anticancer activities of toxic isolates of X. testudinaria sponge from Sanur, Bali, Indonesia, and identify the responsible compounds.
Materials and Methods
Ethical approval
The study only used invertebrate, so ethical approval was not necessary.
Materials
The X. testudinaria sponge was collected from the coastal waters of Sanur, Bali, on May 9, 2018. Methanol, n-hexane, chloroform, and n-butanol were purchased from Merck, Germany. Brine shrimp Artemia salina eggs were purchased from American Technology. The cell line was purchased from the Primate Study Centre, Bogor Agriculture University. Gas chromatography-mass spectrometry (GC-MS) was performed using a GC-MS-QP2010 Ultra Shimadzu from Japan.
Sample preparation and extraction
Fresh sponge samples were washed with water until clean, cut into small pieces, and dried away from direct sunlight for 7 days. After drying, the sample was sieved through at 100 mesh filter to ensure appropriate homogeneity. In total, 500 g dry powder of sample was extracted using methanol maceration. Every 24 h, the extract was filtered, and the pulp was re-extracted using fresh methanol. This extraction process was conducted 3 times. All the methanol extracts were evaporated using a rotary evaporator to yield the crude extract [19].
Fractionation
The crude extract (10 g) was completely dissolved in a methanol-water mixture (3:7), and then the methanol was removed by evaporation. The water extract was fractioned successively with n-hexane (3×100 mL), chloroform (3×100 mL), and n-butanol (3×100 mL). The solvents were removed by evaporation to obtain the EH, chloroform extract (EC), and n-butanol extract (EB). All three extracts (EH, EC, and EB) were tested for toxicity [19].
Separation and purification
The most toxic extracts were then separated by silica gel column chromatography using suitable eluents to obtain several fractions. All fractions were tested for toxicity. The most toxic fraction was tested for purity by thin-layer chromatography (TLC) using several eluent systems. If the isolate provided a single stain on the TLC plate in various eluent systems, then the isolate was considered pure according to TLC; finally, the anticancer effect of the isolate was determined in HeLa cells [19].
Toxicity test
The medium for larvae hatching was made by filtering seawater. Seawater was placed in an aquarium, which was divided into two parts; one was dark and the other was bright. A. salina eggs (50 mg) were placed or immersed in the dark part and left for 48 h until it hatched into a mature larva and was ready to use for testing. Each methanol and n-EH (20 mg) were dissolved into 2 mL of solvent. These solutions were considered as 500 μL, 50 μL, and 5 μL, respectively; each solution was inserted into the test tube, and the solvent was evaporated. Dimethyl sulfoxide (nearly 50 μL), seawater (1 mL), and 10 larvae were placed into a test tube containing the sample; the solvent was evaporated, and seawater was added to a volume of 5 mL, to obtain extract concentrations of 1000, 100, and 10 ppm. A concentration of 0 ppm (solution without the addition of the extract) was prepared as a control. After 24 h, the death of A. saline larvae was measured. The standard assessment of larval mortality is when the larvae do not show movement during an observation period of several seconds [28]. The number of live and dead larvae was recorded, and the data were analyzed to find the LC50.
Anticancer test
The toxic isolate was assayed for its anticancer activity against HeLa cells [29]. HeLa cells were cultured in Roswell Park Memorial Institute 1640 medium, and the initial number of cells was counted using a microscope. The cells were trypsinized, harvested, and centrifuged to form two layers (sediment and supernatant). The supernatant was removed and the precipitate was pelletized; 1 mL of complete medium was added and then the number of cells as counted using a hemocytometer. Subsequently, 2×104 cells were seeded in 100 μL of the medium in a 96-well plate and incubated for 1–2 h to allow the cells to adhere. Subsequently, 100 µL extracts of the test material were added at various concentrations (1000, 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.91, 1.95, 0.97, 0.48, 0.24, 0.12, and 0.06 μg/mL), to make a total volume of 200 µL in each well. The cells were then incubated for 24 h at 37°C. After 24 h, the cells were observed using the microscope. 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 μg/mL) was added to each and incubated for 4 h. Subsequently, stop solution of sodium dodecyl sulfate (SDS) 10% in 0.01 N HCl was added into each well and incubated overnight. The absorbance at 500 nm was observed using an enzyme-linked immunosorbent assay plate reader.
Identification of compounds
Anticancer compounds were separated using GC-MS. The mass spectrum of the active isolates obtained was compared with standard reference spectra that were programmed on the device (GC-MS-QP2010 Ultra Shimadzu) [30,31].
Anticancer activity
Anticancer activity against HeLa cells was determined using the MTT assay. The MTT assay is a colorimetric method used to measure cell proliferation. The principle of the assay is the reduction of yellow tetrazolium salt MTT, which is reduced to purple formazan crystals by living mitochondria [32]: MTT is absorbed into live cells and reduced by succinate dehydrogenase in the electron transport chain of mitochondria to formazan. The formed formazan crystals are dissolved in 10% SDS, forming a purple solution. The MTT reduction occurs through the NADH and NADPH cofactor pyridine nucleotides, which only occur in living cells; thus, the amount of formazan formed is proportional to the number of live cells [33]. The optical density (OD) of each well was measured at 595 nm using a microplate reader. All tests were conducted in triplicate, and the average OD value was converted to a percentage inhibition.
Results
Sample preparation and extraction
Fresh sample (10 kg) was washed, cut, and dried for 7 days, which produced 953 g of dried samples. The dried samples were refined to produce 781 g of dry sample powder. Subsequently, 500 g of dry sample powder was extracted using methanol, which yielded 67 g of the methanol extract.
Fractionation
The methanol extract (50 g) was partitioned successively with n-hexane, chloroform, and n-butanol to produce the n-hexane, chloroform, and n-EB, respectively, with yields of 5.17, 3.84, and 9.27 g, respectively. The results of the toxicity test of the three extracts are shown in Table-1. The EC was the most toxic, with an LC50 value of 39.81 ppm.
Table 1.
Toxicity of n-hexane, chloroform, and n-butanol extracts.
| Sample | Concentration (ppm) | Number of dead larvae | Mortality (%) | LC50 (ppm) | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| EH | 0 | 0 | 0 | 0 | 0 | 70.79 |
| 10 | 1 | 1 | 2 | 7 | ||
| 100 | 5 | 6 | 6 | 58 | ||
| 1000 | 10 | 9 | 9 | 94 | ||
| EC | 0 | 0 | 0 | 0 | 0 | 39.81 |
| 10 | 2 | 1 | 1 | 8 | ||
| 100 | 7 | 8 | 8 | 75 | ||
| 1000 | 10 | 9 | 10 | 95 | ||
| EB | 0 | 0 | 0 | 0 | 0 | 63.09 |
| 10 | 2 | 2 | 1 | 13 | ||
| 100 | 5 | 6 | 7 | 58 | ||
| 1000 | 10 | 9 | 10 | 100 | ||
EH=Hexane extract, EC=Chloroform extract, EB=N-butanol extract
Separation and purification
The EC was separated by silica gel column chromatography using n-hexane-chloroform (1.5:8.5) as an eluent, which produced four fractions (Fraction A, Fraction B [FB], Fraction C, and Fraction D). The toxicity data of the four fractions are shown in Table-2.
Table 2.
The toxicity of the fraction results from column chromatography.
| Sample | Concentration (ppm) | Number of dead larvae | Mortality (%) | LC50 (ppm) | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| FA | 0 | 0 | 0 | 0 | 0 | 50.12 |
| 10 | 2 | 1 | 1 | 7 | ||
| 100 | 7 | 7 | 6 | 67 | ||
| 1000 | 10 | 9 | 9 | 94 | ||
| FB | 0 | 0 | 0 | 0 | 0 | 44.67 |
| 10 | 2 | 1 | 1 | 8 | ||
| 100 | 7 | 7 | 6 | 73 | ||
| 1000 | 10 | 10 | 9 | 100 | ||
| FC | 0 | 0 | 0 | 0 | 0 | 56.23 |
| 10 | 2 | 2 | 1 | 13 | ||
| 100 | 7 | 6 | 6 | 62 | ||
| 1000 | 10 | 9 | 9 | 94 | ||
| FD | 0 | 0 | 0 | 0 | 0 | 79.43 |
| 10 | 2 | 2 | 1 | 13 | ||
| 100 | 5 | 5 | 6 | 54 | ||
| 1000 | 10 | 9 | 9 | 94 | ||
FA=Fraction A, FB=Fraction B, FC=Fraction C, FD=Fraction D
As shown in Table-2, the most toxic fraction was FB, which had an LC50 value of 44.67 ppm. The FB isolate was then tested for purity using the silica gel TLC method. The tests with various eluent systems all resulted in a single stain, which indicated that the FB isolate was pure, according to TLC.
Anticancer activity
The OD data and percentage inhibition of HeLa cells after treatment with sponge X. testudinaria toxic isolate (FB) are presented in Table-3.
Table 3.
Toxic isolate (FB) inhibition.
| Sample (ppm) | OD | Average | Inhibition (%) | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| 100 | 0.046 | 0.045 | 0.25 | 0.038 | 80.8a |
| 50 | 0.052 | 0.046 | 0.032 | 0.043 | 78.28b |
| 25 | 0.062 | 0.056 | 0.03 | 0.049 | 75.25c |
| 12.5 | 0.071 | 0.056 | 0.04 | 0.056 | 71.71d |
| 6.25 | 0.075 | 0.063 | 0.07 | 0.069 | 65.15e |
| 3.125 | 0.098 | 0.083 | 0.09 | 0.090 | 54.54f |
| 1.56 | 0.102 | 0.1 | 0.1 | 0.100 | 49.49g |
| 0.78 | 0.125 | 0.115 | 0.117 | 0.119 | 39.89h |
| 0.39 | 0.139 | 0.140 | 0.150 | 0.143 | 27.77i |
| 0.195 | 0.145 | 0.155 | 0.160 | 0.153 | 22.72j |
| Cell control | 0.195 | 0.197 | 0.202 | 0.198 | 0.00k |
*Values followed by the same letters in the same column are not significantly different according to the Duncan’s Multiple Range Test at p<5%. FB = Fraction B, OD = Optical density
Based on the data in Table-3, the relationship between sample concentration and inhibition was used to determine the IC50 (Figure-1).
Figure-1.
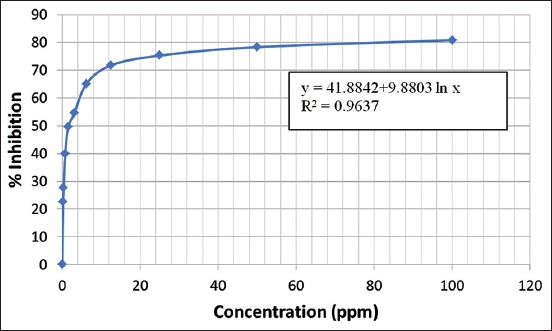
The curve correlation between sample concentration and inhibition.
The equation of the graph in Figure-1 is y = 47.4729+8.9399ln(x), and the coefficient determination (R2) was 0.9393. Therefore, the IC50 was calculated from the following equation:
50 = 41.8842+9.8803ln(x)
ln(x) = (50-41.8842)/9.8803 = 0.821412
x = 2.273
The IC50 of the toxic isolate from FB was 2.273 ppm, which is considered a very strong activity [34].
Identification of compounds
The GC analysis of the anticancer is presented in Figure-2. Twenty-one compounds were present in the isolate. The compounds were identified by MS and are presented in Table-4.
Figure-2.
Gas chromatogram of the anticancer isolate of Xestospongia testudinaria sponge.
Table 4.
Compounds identified in the anticancer isolate of X. testudinaria sponge.
| Peak | Retention time (min) | Abundance (%) | Molecular weight | Molecular formula | Compound name |
|---|---|---|---|---|---|
| 1 | 13.283 | 13.66 | 188 | C9H16O4 | Nonanedioic acid |
| 2 | 20.167 | 15.37 | 228 | C14H28O2 | Tetradecanoic acid |
| 3 | 21.917 | 2.77 | 296 | C20H40O | Trans-phytol |
| 4 | 22.067 | 2.22 | 268 | C18H36O | 2-Pentadecanone-6,10,14-trimethyl |
| 5 | 22.350 | 0.99 | 242 | C15H30O2 | Pentadecanoic acid |
| 6 | 22.850 | 2.02 | 278 | C20H38 | 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl- |
| 7 | 23.575 | 0.43 | 170 | C11H22O | 2,3,7-Trimethyloctanal |
| 8 | 24.583 | 50.24 | 256 | C16H32O2 | Palmitic acid |
| 9 | 25.150 | 1.50 | 368 | C24H48O2 | Docosanoic acid, ethyl ester |
| 10 | 25.717 | 0.75 | 248 | C18H32 | 1, E-11, Z-13-octadecatriene |
| 11 | 27.458 | 0.88 | 282 | C13H24Cl2O2 | Chloromethyl 4-chlorododecanoate |
| 12 | 29.017 | 1.04 | 322 | C23H46 | 1-Tricosene |
| 13 | 30.183 | 0.48 | 294 | C19H34O2 | 9,12-Octadecadienoic acid |
| 14 | 32.000 | 1.39 | 324 | C21H40O2 | 4,8,12,16-Tetramethylheptadecan-4-olide |
| 15 | 32.550 | 0.57 | 308 | C22H44 | 1-Docosene |
| 16 | 34.292 | 0.72 | 296 | C21H44 | Heneicosane |
| 17 | 35.817 | 0.69 | 586 | C36H75O3P | Phosphonic acid, dioctadecyl ester |
| 18 | 35.892 | 1.12 | 198 | C14H30 | Dodecane, 4,6-dimethyl- |
| 19 | 37.417 | 1.52 | 478 | C34H70 | n-Tetratriacontane |
| 20 | 38.900 | 0.87 | 352 | C16H33I | 1-Iodohexadecane |
| 21 | 40.450 | 0.79 | 296 | C21H44 | n-Heneicosane |
X. testudinaria=Xestospongia testudinaria
Discussion
The data presented in Tables-1 and 2 show that the cytotoxic potency of X. testudinaria sponge extract increased from the EC (39.81 ppm) to FB (44.67 ppm). This indicated that the toxic compounds in the EC exerted a synergistic effect [35]. Based on the anticancer preliminary test (toxicity test), the EC was the most toxic extract, indicating that the toxic compounds are semipolar because they dissolve into chloroform (Table-2) [36]. As shown in Figure-1, the sample concentration was positively correlated with the inhibition of HeLa cell growth. All inhibition percentages of the samples and concentrations were significantly different from the control. Compared with a previous report [19], the anticancer activity of toxic isolates was lower, with an IC50 of 2273 ppm. This showed that the anticancer compounds in the X. testudinaria sponge exerted synergistic effects.
The anticancer activity (IC50 = 2.273 ppm) of X. testudinaria sponge toxic isolate agreed with to a previous report [18], in which the IC50 of ethanol extract of X. testudinaria sponge on HeLa, HepG-2, and Daoy cells was 83.35, 23.45, and 23.31 ppm, respectively. In addition, the n-EH from the same sponge had IC50 values of 33.7, 30.2, and 20.74 ppm in of on HeLa, HepG-2, and Daoy cells, respectively.
We found some compounds of anticancer isolates in Table-4 were fatty acids and esters (nonanedioic acid, tetradecanoic acid, pentadecanoic acid, palmitic acid, docosanoic acid, ethyl ester, 9,12-octadecadienoic acid, phosphonic acid, and dioctadecyl ester). The derivatives of phenyl oleic acid are known to inhibit the growth of MCF-7 and HT-29 cancer cells, with IC50 values of 48 ppm, whereas n-butyl oleic acid derivatives inhibited the growth of these cells with IC50 values of 82 ppm and 77 ppm, respectively [37]. The anticancer activity of ω-6 polyunsaturated fatty acids is known [38]. Compounds isolated from Cladophora fracta, such as oleic acid, palmitic acid, gamma-linolenic acid, and linoleic acid, are reported to have strong antiproliferative activity [39]. Anticancer properties are also found in palmitic acid, (Z)-9-octadecenoic acid, and octadecenoic acid isolated from Protaetia brevitarsis larvae [40].
Terpenoid compounds were also detected in anticancer isolates from X. testudinaria sponge; namely, trans phytol, 1-tricosene, and 2-hexadecen-1-ol, 3,7,11,15-tetramethyl-. These results were in accordance with previous publications, which reported that phytol and diterpene alcohol compounds had anticancer activities in MCF-7 and PC-3 cells, with IC50 values of 8.79±0.41 μM and 77.85±1.93 μM, respectively [41]. Other phytol and diterpenes were isolated from Justicia gendarussa Burm. f. used as an anti-inflammatory, with histamine release (26.92%), serotine and bradykinin (49.90%), and prostaglandin (68.03%) as compared with the standard (Diclofenac 5 mg/kg) [42]. Phytol also shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane [43,44].
Hydrocarbons (4,8,12,16-tetramethylheptadecan-4-olide; 1-docosene; heneicosane; n-heneicosane) were detected in the anticancer isolate of X. testudinaria sponge. Pyrenyl ether, which is a polycyclic aromatic compound with a good cytotoxic effect against cisplatin-induced colon cancer cells (HT-29) and HeLa cancer cells [45]. Essential oil from Gannan navel orange peel, which contains linalool, 3-carene, α-terpineol, decanal, citral, D-limonene, and α-pinene, is reported to have anticancer activity against Hela cells [46]. The sterol fraction of Taonia atomaria can inhibit cancer cells: HePG2, A549, HCT116, and MCF7 [47]. The antitumor activity of saturated aliphatic hydrocarbons in Pyrostegia venusta, namely, octasane and triacontane compounds from the heptane extract, has also been reported [48].
Conclusion
The anticancer activity of X. testudinaria sponge toxic isolates was positively correlated with the inhibition of HeLa cell growth, with an IC50 of 2.273 ppm. In total, 21 compounds were identified in toxic isolate; three groups of compounds were identified, namely, fatty acids and esters, terpenoids, and hydrocarbons.
Authors’ Contributions
MDS designed and conducted the experiment, acquisition of data, and drafting of the manuscript. WSR conducted the experiment and collected the data. NS designed the experiment, drafting of the manuscript. KKA analyzed the data and drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors wish to express their gratitude to the Directorate of Research and Community Service, Directorate-General for Research and Development, Ministry of Research, Technology and Higher Education of the Republic of Indonesia that have funded this research through National strategic research Institution Grant Year 2018 with Contract No. 171.85/UN14.4.A/LT/2018. The authors would also like to thank the Institute for Research and Community Services, Udayana University, which has been facilitated this research.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Hejmadi M. Introduction to Cancer Biology. 2nd ed. Bookboon, Denmark: 2010. [Google Scholar]
- 2.Martin T.A, Ye L, Sanders A.J, Lane J, Jiang W.G. Cancer Invasion and Metastasis:Molecular and Cellular Perspective. Austin: Landes Biosci; 2013. pp. 1–34. [Google Scholar]
- 3.Zaorsky N.G, Churilla T.M, Egleston B.L, Fisher S.G, Ridge J.A, Horwitz E.M, Meyer J.E. Causes of death among cancer patients. Ann. Oncol. 2017;28(2):400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Cancer, Fact Sheets. 2018. [Last accessed on 31-03-2019]. Available from: http://www.who.int/news-room/fact-sheets/detail/cancer .
- 5.Chakraborty S, Rahman T. The difficulties in cancer treatment. Ecancermedicalscience. 2012;6(16):1–5. doi: 10.3332/ecancer.2012.ed16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Human Papilloma Virus (HPV) and Cervical Cancer. 2013. [Last accessed on 31-03-2019]. Available from: http://www.who.int/mediacentre/factsheets/fs380/en.
- 7.Edianto D. Cervical Cancer. Jakarta: Bina Pustaka Foundation Sarwono Prawirahardjo; 2006. [Google Scholar]
- 8.Abeloff M, Arnitage J, Niederhuber J, Kastan M, McKenna W. Clinical Oncology. 3rd ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2004. [Google Scholar]
- 9.Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors:Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018;7(11):1–20. doi: 10.1186/s40169-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boopathy S.N, Kathiresan K. Anticancer drugs from marine flora:An overview. J. Oncol 2010. 2010;214186:1–18. doi: 10.1155/2010/214186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trianto A, Ambariyanto Murwani R. Skrining bahan anti kanker pada berbagai jenis sponge dan gorgonian terhadap L1210 cell line. Ilmu Kelautan. 2004;9(3):120–124. [Google Scholar]
- 12.Munro M.H.G, Luibrand R.T, Blunt J.W. The search for antiviral and anticancer compounds from marine organisms. Bioorganic. Med. Chem. 1989;1(1):94–176. [Google Scholar]
- 13.Soediro I.S. Marine Biological Products and Prospects for Utilization in Health and Cosmetics. Proceeding Indonesian Marine Biotechnology Seminar I. Jakarta. 1999:41–52. [Google Scholar]
- 14.Soest R.W.M, Braekman J.C. Chemosystematics of porifera:A review. Mem. Qld. Mus. 1999;44(1-2):569–589. [Google Scholar]
- 15.Hassan W.H.B. Isolation and Structure Elucidation of Bioactive Secondary Metabolites from Marine Sponges. Thesis Dissertation Heinrich Heine, Düsseldorf. 2004 [Google Scholar]
- 16.Hanani E, Mun'im A, Sekarini R. Identifikasi senyawa antioksidan dalam spons Callyspongia spp. dari kepulauan seribu. Majalah Ilmu Kefarmasian. 2005;2(3):127–133. [Google Scholar]
- 17.Kobayashi M, Rachmaniar R. Overview of Marine Natural Product Chemistry. Proceeding Indonesian Marine Biotechnology Seminar I. Jakarta. 1999:23–32. [Google Scholar]
- 18.El-Gamal A.A, Al-Massarani S.M, Shaala L.A, Alahdald A.M, Al-Said M.S, Ashour A.E, Kumar A, Abdel-Kader M.S, Abdel-Mageed W.M, Youssef D.T. Cytotoxic compounds from the Saudi red sea sponge Xestospongia testudinaria. Mar. Drugs. 2016;14(5):1–9. doi: 10.3390/md14050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swantara I.M.D, Rita W.S, Suartha I.N. Anticancer activities of toxic extract of Xestospongia testudinaria sponge from Sanur Beach, Bali, Indonesia. Res. J. Pharm. Biol. Chem. Sci. 2018;9(3):1036–1041. [Google Scholar]
- 20.Ayyad S.E.N, Katoua D.F, Alarif W.M, Sobahi T.R, Aly M.M, Shaala L.A, Ghandourah M.A. Two new polyacetylene derivatives from the red sea sponge Xestospongia spp. Z. Naturforsch. C. 2015;70(11-12):297–303. doi: 10.1515/znc-2015-5015. [DOI] [PubMed] [Google Scholar]
- 21.Bourguet-Kondracki M.L, Rakotoarisoa M.T, Martin M.T, Guyot M. Bioactive bromopolyacetylenes from the marine sponge Xestospongia testudinaria. Tetrahedron Lett. 1992;33(2):225–226. [Google Scholar]
- 22.Cita Y.P, Suhermanto A, Radjasa O.K, Sudharmono P. Antibacterial activity of marine bacteria isolated from sponge Xestospongia testudinaria from Sorong, Papua. Asian Pac. J. Trop. Biomed. 2017;7(5):450–454. [Google Scholar]
- 23.Nguyen X.C, Longeon A, Pham V.C, Urvois F, Bressy C, Trinh T.T.V, Nguyen H.N, Phan V.K, Chau V.M, Briand J.F, Bourguet-Kondracki M.L. Antifouling 26, 27-cyclosterols from the Vietnamese marine sponge Xestospongia testudinaria. J. Nat. Prod. 2013;76(7):1313–1318. doi: 10.1021/np400288j. [DOI] [PubMed] [Google Scholar]
- 24.Zhou C, Yuan K, Tang X, Hu N, Peng W. Molecular genetic evidence for polyandry in Ascaris suum. Parasitol. Res. 2011;108(3):703–708. doi: 10.1007/s00436-010-2116-3. [DOI] [PubMed] [Google Scholar]
- 25.Swantara I.M.D, Rita W.S. Toxicity of sponge extract Xestospongia testudinaria. Int. J. Eng. Sci. Invent. 2018;7(5):55–58. [Google Scholar]
- 26.Meyer B.N, Ferrigni N.R, Putnam J.E, Jacobsen L.B, Nicholas D.E, Mclaughlin J.L. Birne shrimp:A convenient general bioassay for active plant constituents. J. Med. Plant Res. 1982;45(1):31–34. [PubMed] [Google Scholar]
- 27.Ekowati H, Astuti I, Mustofa M. Anticancer activity of calanone on HeLa cell line. Indones. J. Chem. 2010;10(2):247–251. [Google Scholar]
- 28.Carballo J.L, Hernández-Inda Z.L, Pérez P, García-Grávalos M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002;2(17):1–5. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCauley J, Zivanovic A, Skropeta D. Bioassays for anticancer activities. Methods Mol. Biol. 2013;1055(1):191–205. doi: 10.1007/978-1-62703-577-4_14. [DOI] [PubMed] [Google Scholar]
- 30.Byju K, Anuradha V, Vasundhara G, Nair S.M, Kumar C.N. In vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral Subergorgia reticulata. Pharmacogn. Mag. 2014;10(Suppl 1):S65–S71. doi: 10.4103/0973-1296.127345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganesh M, Mohankumar M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography-mass spectrometry. J. Food Sci. Technol. 2017;54(10):3082–3091. doi: 10.1007/s13197-017-2744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Doyle A, Griffiths J.B. Cell and Tissue Culture for Medical Research. New York: John Wiley and Sons Ltd; 2000. [Google Scholar]
- 34.Chao S.G, Valerie H.L, Wu X.H, Sim K.Y, Tan B.H.K, Pereira J.T. Novel cytotoxic olyprenylated xanthones from Garcinia gaudichaudii. Tetrahedron. 1998;54(36):10915–10924. [Google Scholar]
- 35.Machana S, Weerapreeyakul N, Barusrux S, Thumanu K, Tanthanuch W. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac. J. Trop. Biomed. 2012;2(8):589–596. doi: 10.1016/S2221-1691(12)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakhmawati R, Anggarwulan E, Retnaningtyas E. Potency of lobak leaves (Raphanus sativus L. var. Hortensis back) as anticancer and antimicrobial candidates. Biodiversitas. 2009;10(3):158–162. [Google Scholar]
- 37.Dailey O.D.J, Wang X, Chen F, Huang G. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer Res. 2011;31(10):3165–3169. [PubMed] [Google Scholar]
- 38.Xu Y, Qian S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014;37(3):112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karan T, Erenler R. Fatty acid constituents and anticancer activity of Cladophora fracta (OF Müller ex Vahl) kützing. Trop. J. Pharm. Res. 2018;17(10):1977–1982. [Google Scholar]
- 40.Yoo Y.C, Shin B.H, Hong J.H, Lee J, Chee H.Y, Song K.S, Lee K.B. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis larva. Arch. Pharm. Res. 2007;30(3):361–365. doi: 10.1007/BF02977619. [DOI] [PubMed] [Google Scholar]
- 41.Pejin B, Kojic V, Bogdanovic G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014;28(22):2053–2056. doi: 10.1080/14786419.2014.921686. [DOI] [PubMed] [Google Scholar]
- 42.Phatangare N.D, Deshmukh K.K, Murade V.D, Hase G.J, Gaje T.R. Isolation and characterization of phytol from Justicia gendarussa Burm. f. An anti-inflammatory compound. Int. J. Pharmacogn. Phytochem. Res. 2017;9(6):864–872. [Google Scholar]
- 43.Jeong S.H. Inhibitory effect of phytol on cellular senescence. Biomed. Dermatol. 2018;2(13):1–9. [Google Scholar]
- 44.Sakthivel R, Malar D.S, Devi K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018;105(2018):742–752. doi: 10.1016/j.biopha.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Bandyopadhyay D, Granados J.C, Short J.D, Banik B.K. Polycyclic aromatic compounds as anticancer agents:Evaluation of synthesis and in vitro cytotoxicity. Oncol. Lett. 2012;3(1):45–49. doi: 10.3892/ol.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Chen H, Chen H, Zhong B, Luo X, Chun J. Antioxidant and anticancer activities of essential oil from gannan navel orange peel. Molecules. 2017;22(1391):1–10. doi: 10.3390/molecules22081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim E.A, Aly H.F, Baker D.H.A, Mahmoud K, El-Baz F.K. Marine algal sterol hydrocarbon with anti-inflammatory, anticancer and antioxidant properties. Int. J. Pharm. Bio. Sci. 2016;7(3):392–398. [Google Scholar]
- 48.Figueiredo C.R, Matsuo A.L, Pereira F.V, Rabaca A.N, Farias C.F, Girola N, Massaoka M.H, Azevedo R.A, Scutti J.A, Arruda D.C, Silva L.P, Rodrigues E.G, Lago J.H, Travassos L.R, Silva R.M. Pyrostegia venusta heptane extract containing saturated aliphatic hydrocarbons induces apoptosis on B16F10-Nex2 melanoma cells and displays antitumor activity in vivo. Pharmacogn. Mag. 2014;10(38):S363. doi: 10.4103/0973-1296.133284. [DOI] [PMC free article] [PubMed] [Google Scholar]


