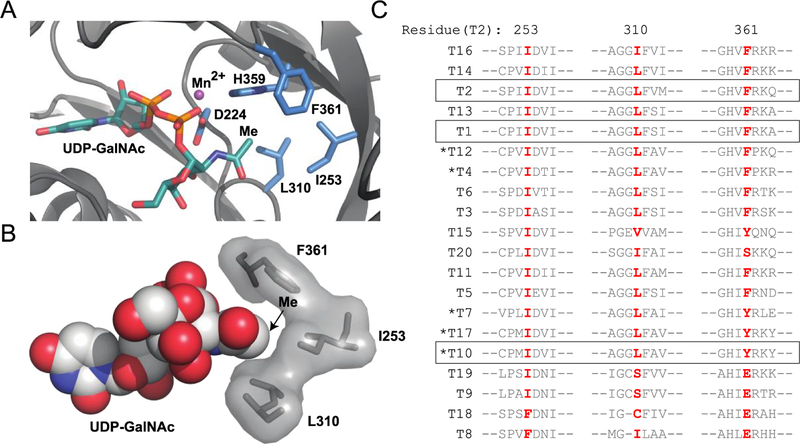
Figure 1.
Identification of gatekeeper residues. (A) Residues within 5 Å of GalNAc methyl carbon for GalNAc-T2 (PDB ID 4D0T). Five of seven amino acids in close proximity to the GalNAc methyl contain side chains; of these, H359 and D224 coordinate Mn2+, while I253, L310, and F361 are promising hydrophobic residues. (B) Space-filling model of gatekeeper residues within 5 Å of GalNAc methyl in GalNAc-T2 (PDB ID 4D0T). (C) Amino acid sequences of human GalNAc-T1–GalNAc-T20 surrounding potential gatekeeper residues demonstrate a high degree of conservation with 13 isoenzymes containing Ile/Leu and 18 containing Ile at residues homologous to GalNAc-T2 positions 253/310 or 253, respectively. Only GalNAc-T8 and -T18 have dramatically different residues at positions corresponding to 253 and around 310 of GalNAc-T2. GalNAc-Ts used in this study are boxed. Clustal Omega was used to generate a multiple sequence alignment of the amino acid sequences corresponding to the full-length genes of human GalNAc-T1-GalNAc-T20 (Figure S2; Table S1). GalNAc-Ts are ordered based on homology, and GalNAc-Ts that predominantly prefer GalNAc-peptides are denoted with an asterisk.10