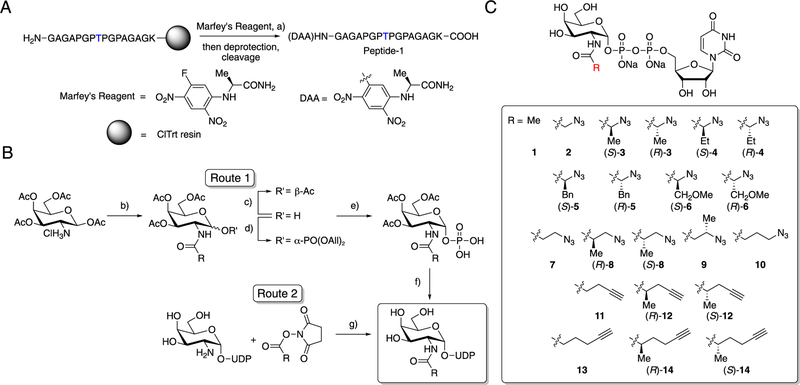
Figure 2.
Synthesis of a peptide substrate and a panel of UDP-GalNAc analogs. (A) Synthetic route for Peptide-1. Blue T indicates the Thr glycosylation site used by GalNAc-T2. (B) Synthetic routes for UDP-GalNAc analogs. Route 1 was used to synthesize UDP-sugars ((S)-3, (R)-3, (S)-4, (R)-4, (S)-5, (R)-5, (S)-6, (R)-6, (S)-8, (R)-8, (S)-12, (R)-12, (S)-14, (R)-14), and Route 2 was used to synthesize UDP-sugars (2, 7, 9, 10,11, 13). (C) Panel of UDP-GalNAc derivatives with azide or alkyne chemical handles. Compounds 1 and 2 are the natural substrate UDP-GalNAc and known analog UDP-GalNAz, respectively. Reagents and conditions: (a) NEt(i-Pr)2, DMF, rt; (b) R-COOH, COMU, NEt(i-Pr)2, DMF, 0 °C to rt; (c) N,N′-dimethyl-1,3-diaminopropane, THF, rt; (d) i-Pr2NPO(OAll)2, 1H-tetrazole, CH2Cl2, 0 °C, then m-CPBA, −78 °C; (e) Pd(PPh3)4, sodium p-toluenesulfinate, THF/MeOH, rt; (f) (i) uridine 5′-monophosphomorpholidate 4-morpholine-N,N′-dicyclohexylcarboxamidine salt, 1-methylimidazole hydrochloride, NEt3, DMF, rt; (ii) MeOH/water/NEt3, rt; (g) HEPES buffer (pH = 8.0), 0 °C to rt.