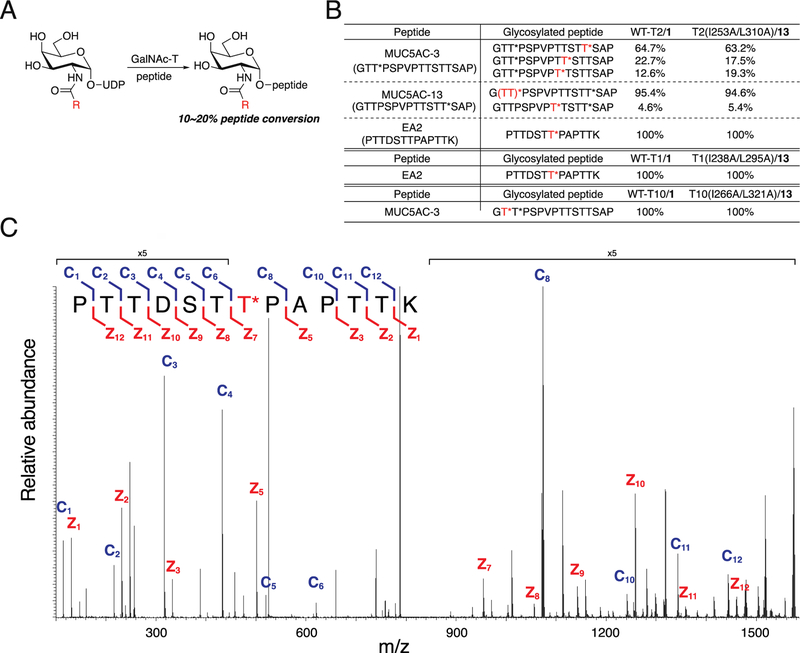
Figure 6.
Glycosylation of natural peptide substrates by wild-type and engineered GalNAc-T isoenzyme−substrate pairs. (A) Scheme for glycosylation reaction with GalNAc-T, natural peptide substrate, and UDP-GalNAc or UDP-GalNAc analog to form glycosylated peptide. Glycosylation reactions were terminated at 10−20% glycopeptide formation. (B) Percent of glycosylated peptide formed out of total glycosylated peptide formed. Reactions were performed as in A at 37 °C and quenched by addition of aqueous EDTA (150 mM, pH = 8.0). Naturally occurring glycopeptides MUC5AC-3 and MUC5AC-13 each contain a single GalNAc-O-Thr (T*). Red T* indicates the site of glycosylation by the GalNAc-T of interest. Glycosylation of MUC5AC-3 by GalNAc-T2 yielded a major product that was glycosylated at Thr3 by wild-type GalNAc-T2/1. The glycosite from T2(I253A/L310A)/13 could not be unambiguously assigned and was either Thr2 or Thr3, labeled (TT)*. (C) Representative MS/MS spectrum of EA2 glycosylated by T2(1253A/L310A)/13 upon fragmentation and sequencing. Fragmentation pattern of EA2 amino acid sequence to generate c ions (blue) and z ions (red) is shown.